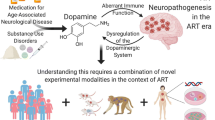
Abstract
Although the incidence of HIV-associated dementia (HAD) has declined, HIV-associated neurocognitive disorders (HAND) remain a significant health problem despite use of highly active antiretroviral therapy. In addition, the incidence and/or severity of HAND/HAD are increased with concomitant use of drugs of abuse, such as cocaine, marijuana, and methamphetamine. Furthermore, exposure to most drugs of abuse increases brain levels of dopamine, which has been implicated in the pathogenesis of HIV. This review evaluates the potential role of dopamine in the potentiation of HAND/HAD by drugs of abuse. In the brain, multiplication of HIV in infected macrophages/microglia could result in the release of HIV proteins such as gp120 and Tat, which can bind to and impair dopamine transporter (DAT) functions, leading to elevated levels of dopamine in the dopaminergic synapses in the early asymptomatic stage of HIV infection. Exposure of HIV-infected patients to drugs of abuse, especially cocaine and methamphetamine, can further increase synaptic levels of dopamine via binding to and subsequently impairing the function of DAT. This accumulated synaptic dopamine can diffuse out and activate adjacent microglia through binding to dopamine receptors. The activation of microglia may result in increased HIV replication as well as increased production of inflammatory mediators such as tumor necrosis factor (TNF)-alpha and chemokines. Increased HIV replication can lead to increased brain viral load and increased shedding of HIV proteins, gp120 and Tat. These proteins, as well as TNF-alpha, can induce cell death of adjacent dopaminergic neurons via apoptosis. Autoxidation and metabolism of accumulated synaptic dopamine can lead to generation of reactive oxygen species (hydrogen peroxide), quinones, and semiquinones, which can also induce apoptosis of neurons. Increased cell death of dopaminergic neurons can eventually lead to dopamine deficit that may exacerbate the severity and/or accelerate the progression of HAND/HAD.


Similar content being viewed by others
References
Clifford DB (2008) HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med 16:94–98
Anthony IC, Bell JE (2008) The neuropathology of HIV/AIDS. Int Rev Psychiatr 20:15–24
Dore GJ, Correll PK, Li Y, Kaldor JM, Cooper DA, Brew BJ (1999) Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS 13:1249–1253
Thomas SA (2004) Anti-HIV drug distribution to the central nervous system. Curr Pharm Des 10:1313–1324
Davies J, Everall IP, Weich S, McLaughlin J, Scaravilli F, Lantos PL (1997) HIV-associated brain pathology in the United Kingdom: an epidemiological study. AIDS 11:1145–1150
Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR (2001) Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol 7:66–71
Cristiani SA, Pukay-Martin ND, Bornstein RA (2004) Marijuana use and cognitive function in HIV-infected people. J Neuropsychiatry Clin Neurosci 16:330–335
Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I, HNRC Group (2004) Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc 10:1–14
Burdo TH, Katner SN, Taffe MA, Fox HS (2006) Neuroimmunity, drugs of abuse, and neuroAIDS. J Neuroimmune Pharmacol 1:41–49
Ferris MJ, Mactutus CF, Booze RM (2008) Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev 32:883–909
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–5278
Pontieri FE, Tanda G, Di Chiara G (1995) Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A 92:12304–12308
Bradberry CW (2000) Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine use. J Neurosci 20:7109–7115
Walker QD, Kuhn CM (2008) Cocaine increases stimulated dopamine release more in periadolescent than adult rats. Neurotoxicol Teratol 30:412–418
Brody AL, London ED, Olmstead RE, Allen-Martinez Z, Shulenberger S, Costello MR, Abrams AL, Scheibal D, Farahi J, Shoptaw S, Mandelkern MA (2010) Smoking-induced change in intrasynaptic dopamine concentration: effect of treatment for tobacco dependence. Psychiatr Res 183:218–224
Czub S, Czub M, Koutsilieri E, Sopper S, Villinger F, Müller JG, Stahl-Hennig C, Riederer P, Ter Meulen V, Gosztonyi G (2004) Modulation of simian immunodeficiency virus neuropathology by dopaminergic drugs. Acta Neuropathol 107:216–226
Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M (2009) Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol 15:257–274
Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW (2009) Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol 175:1148–1159
Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, Pearlson GD (1993) Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology 43:2099–2104
Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R (1991) Nigral degeneration in acquired immune deficiency syndrome (AIDS). Acta Neuropathol 82:39–44
Itoh K, Mehraein P, Weis S (2000) Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta Neuropathol 99:376–384
Dal Pan GJ, McArthur JH, Aylward E, Selnes OA, Nance-Sproson TE, Kumar AJ, Mellits ED, McArthur JC (1992) Patterns of cerebral atrophy in HIV-1-infected individuals: results of a quantitative MRI analysis. Neurology 42:2125–2130
Hestad K, McArthur JH, Dal Pan GJ, Selnes OA, Nance-Sproson TE, Aylward E, Mathews VP, McArthur JC (1993) Regional brain atrophy in HIV-1 infection: association with specific neuropsychological test performance. Acta Neurol Scand 88:112–118
Paul R, Cohen R, Navia B, Tashima K (2002) Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev 26:353–359
Marcario JK, Manaye KF, SantaCruz KS, Mouton PR, Berman NE, Cheney PD (2004) Severe subcortical degeneration in macaques infected with neurovirulent simian immunodeficiency virus. J Neurovirol 10:387–399
Fujimura RK, Goodkin K, Petito CK, Douyon R, Feaster DJ, Concha M, Shapshak P (1997) HIV-1 proviral DNA load across neuroanatomic regions of individuals with evidence for HIV-1-associated dementia. J Acquir Immune Defic Syndr Hum Retrovirol 16:146–152
Kure K, Weidenheim KM, Lyman WD, Dickson DW (1990) Morphology and distribution of HIV-1 gp41-positive microglia in subacute AIDS encephalitis. Pattern of involvement resembling a multisystem degeneration. Acta Neuropathol 80:393–400
Kumar AM, Borodowsky I, Fernandez B, Gonzalez L, Kumar M (2007) Human immunodeficiency virus type 1 RNA Levels in different regions of human brain: quantification using real-time reverse transcriptase-polymerase chain reaction. J Neurovirol 13:210–224
Sardar AM, Czudek C, Reynolds GP (1996) Dopamine deficits in the brain: the neurochemical basis of parkinsonian symptoms in AIDS. NeuroReport 7:910
Kieburtz K, Ketonen L, Cox C, Grossman H, Holloway R, Booth H, Hickey C, Feigin A, Caine ED (1996) Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection. Arch Neurol 53:155–158
Rottenberg DA, Sidtis JJ, Strother SC, Schaper KA, Anderson JR, Nelson MJ, Price RW (1996) Abnormal cerebral glucose metabolism in HIV-1 seropositive subjects with and without dementia. J Nucl Med 37:1133–1141
Berger JR, Kumar M, Kumar A, Fernandez JB, Levin B (1994) Cerebrospinal fluid dopamine in HIV-1 infection. AIDS 8:67–71
Larsson M, Hagberg L, Forsman A, Norkrans G (1991) Cerebrospinal fluid catecholamine metabolites in HIV-infected patients. J Neurosci Res 28:406–409
di Rocco A, Bottiglieri T, Dorfman D, Werner P, Morrison C, Simpson D (2000) Decreased homovanilic acid in cerebrospinal fluid correlates with impaired neuropsychologic function in HIV-1-infected patients. Clin Neuropharmacol 23:190–194
Obermann M, Küper M, Kastrup O, Yaldizli O, Esser S, Thiermann J, Koutsilieri E, Arendt G, Diener HC, Maschke M, German Competence Network HIV/AIDS (2009) Substantia nigra hyperechogenicity and CSF dopamine depletion in HIV. J Neurol 256:948–953
Scheller C, Arendt G, Nolting T, Antke C, Sopper S, Maschke M, Obermann M, Angerer A, Husstedt IW, Meisner F, Neuen-Jacob E, Müller HW, Carey P, Ter Meulen V, Riederer P, Koutsilieri E (2010) Increased dopaminergic neurotransmission in therapy-naïve asymptomatic HIV patients is not associated with adaptive changes at the dopaminergic synapses. J Neural Transm 117:699–705
Czub S, Koutsilieri E, Sopper S, Czub M, Stahl-Hennig C, Müller JG, Pedersen V, Gsell W, Heeney JL, Gerlach M, Gosztonyi G, Riederer P, ter Meulen V (2001) Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta Neuropathol 101:85–91
Scheller C, Sopper S, Jenuwein M, Neuen-Jacob E, Tatschner T, Grünblatt E, ter Meulen V, Riederer P, Koutsilieri E (2005) Early impairment in dopaminergic neurotransmission in brains of SIV-infected rhesus monkeys due to microglia activation. J Neurochem 95:377–387
Jenuwein M, Scheller C, Neuen-Jacob E, Sopper S, Tatschner T, ter Meulen V, Riederer P, Koutsilieri E (2004) Dopamine deficits and regulation of the cAMP second messenger system in brains of simian immunodeficiency virus-infected rhesus monkeys. J Neurovirol 10:163–170
Czub M, Czub S, Gosztonyi G, Koutsilieri E, Sopper S, Müller JG, Gerlach M, Riederer P, ter Meulen V (1999) Effects of selegiline in a retroviral rat model for neurodegenerative disease. J Neurovirol 5:458–464
Rohr O, Sawaya BE, Lecestre D, Aunis D, Schaeffer E (1999) Dopamine stimulates expression of the human immunodeficiency virus type 1 via NF-kappaB in cells of the immune system. Nucleic Acids Res 27:3291–3299
Scheller C, Sopper S, Jassoy C, ter Meulen V, Riederer P, Koutsilieri E (2000) Dopamine activates HIV in chronically infected T lymphoblasts. J Neural Transm 107:1483–1489
Nair MP, Mahajan S, Sykes D, Bapardekar MV, Reynolds JL (2006) Methamphetamine modulates DC-SIGN expression by mature dendritic cells. J Neuroimmune Pharmacol 1:296–304
Nair MP, Saiyed ZM, Nair N, Gandhi NH, Rodriguez JW, Boukli N, Provencio-Vasquez E, Malow RM, Miguez-Burbano MJ (2009) Methamphetamine enhances HIV-1 infectivity in monocyte derived dendritic cells. J Neuroimmune Pharmacol 4:129–139
Liang H, Wang X, Chen H, Song L, Ye L, Wang SH, Wang YJ, Zhou L, Ho WZ (2008) Methamphetamine enhances HIV infection of macrophages. Am J Pathol 172:1617–1624
Sawaya BE, Rohr O, Aunis D, Schaeffer E (1996) Chicken ovalbumin upstream promoter transcription factor, a transcriptional activator of HIV-1 gene expression in human brain cells. J Biol Chem 271:23572–23576
Rohr O, Schwartz C, Aunis D, Schaeffer E (1999) CREB and COUP-TF mediate transcriptional activation of the human immunodeficiency virus type 1 genome in Jurkat T cells in response to cyclic AMP and dopamine. J Cell Biochem 75:404–413
Cami J, Farre D (2003) Drug addiction. N Engl J Med 349:975–986
Volz TJ, Hanson GR, Fleckenstein AE (2007) The role of the plasmalemmal dopamine and vesicular monoamine transporters in methamphetamine-induced dopaminergic deficits. J Neurochem 101:883–888
Seger D (2010) Cocaine, metamfetamine, and MDMA abuse: the role and clinical importance of neuroadaptation. Clin Toxicol (Phila) 48:695–708
Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM (2009) In vivo microdialysis in awake, freely moving rats demonstrates HIV-1 Tat-induced alterations in dopamine transmission. Synapse 63:181–185
Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM (2009) The human immunodeficiency virus-1-associated protein, Tat1-86, impairs dopamine transporters and interacts with cocaine to reduce nerve terminal function: a no-net-flux microdialysis study. Neuroscience 159:1292–1299
Zhu J, Mactutus CF, Wallace DR, Booze RM (2009) HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther 329:1071–1083
Hu S, Sheng WS, Lokensgard JR, Peterson PK, Rock RB (2009) Preferential sensitivity of human dopaminergic neurons to gp120-induced oxidative damage. J Neurovirol 15:401–410
Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS (2004) Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain 127:2452–2458
Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS (2008) Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. NeuroImage 42:869–878
Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237:1219–1223
Amara SG, Kuhar MJ (1993) Neurotransmitter transporters: recent progress. Annu Rev Neurosci 16:73–93
Han DD, Gu HH (2006) Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol 6:6
Reith ME, Li MY, Yan QS (1997) Extracellular dopamine, norepinephrine, and serotonin in the ventral tegmental area and nucleus accumbens of freely moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers. Psychopharmacology (Berl) 134:309–317
Fleckenstein AE, Metzger RR, Wilkins DG, Gibb JW, Hanson GR (1997) Rapid and reversible effects of methamphetamine on dopamine transporters. J Pharmacol Exp Ther 282:834–838
Kokoshka JM, Vaughan RA, Hanson GR, Fleckenstein AE (1998) Nature of methamphetamine-induced rapid and reversible changes in dopamine transporters. Eur J Pharmacol 361:269–275
Metzger RR, Hanson GR, Gibb JW, Fleckenstein AE (1998) 3-4-Methylenedioxymethamphetamine-induced acute changes in dopamine transporter function. Eur J Pharmacol 349:205–210
Brown JM, Hanson GR, Fleckenstein AE (2000) Methamphetamine rapidly decreases vesicular dopamine uptake. J Neurochem 74:2221–2223
Hogan KA, Staal RG, Sonsalla PK (2000) Analysis of VMAT2 binding after methamphetamine or MPTP treatment: disparity between homogenates and vesicle preparations. J Neurochem 74:2217–2220
Brown JM, Hanson GR, Fleckenstein AE (2001) Regulation of the vesicular monoamine transporter-2: a novel mechanism for cocaine and other psychostimulants. J Pharmacol Exp Ther 296:762–767
Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A (2003) Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J Biol Chem 278:12070–12077
Graham DG (1978) Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol 14:633–643
Pedrosa R, Soares-da-Silva P (2002) Oxidative and non-oxidative mechanisms of neuronal cell death and apoptosis by L-3,4-dihydroxyphenylalanine (l-DOPA) and dopamine. Br J Pharmacol 137:1305–1313
Kuhn DM, Francescutti-Verbeem DM, Thomas DM (2006) Dopamine quinones activate microglia and induce a neurotoxic gene expression profile: relationship to methamphetamine-induced nerve ending damage. Ann N Y Acad Sci 1074:31–41
Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM (2001) Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett 305:5–8
Pocernich CB, Sultana R, Mohmmad-Abdul H, Nath A, Butterfield DA (2005) HIV-dementia, Tat-induced oxidative stress, and antioxidant therapeutic considerations. Brain Res Brain Res Rev 50:14–26
Toborek M, Lee YW, Pu H, Malecki A, Flora G, Garrido R, Hennig B, Bauer HC, Nath A (2003) HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. Neurochem 84:169–179
Price TO, Ercal N, Nakaoke R, Banks WA (2005) HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res 1045:57–63
Pocernich CB, Sultana R, Hone E, Turchan J, Martins RN, Calabrese V, Nath A, Butterfield DA (2004) Effects of apolipoprotein E on the human immunodeficiency virus protein Tat in neuronal cultures and synaptosomes. J Neurosci Res 77:532–539
Agrawal L, Louboutin JP, Marusich E, Reyes BA, Van Bockstaele EJ, Strayer DS (2010) Dopaminergic neurotoxicity of HIV-1 gp120: reactive oxygen species as signaling intermediates. Brain Res 1306:116–130
Silvers JM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM (2007) Neurotoxicity of HIV-1 Tat protein: involvement of D1 dopamine receptor. NeuroToxicology 28:1184–1190
Acknowledgments
We are thankful to Dr. Gayathri Dowling for the critical review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Purohit, V., Rapaka, R. & Shurtleff, D. Drugs of Abuse, Dopamine, and HIV-Associated Neurocognitive Disorders/HIV-Associated Dementia. Mol Neurobiol 44, 102–110 (2011). https://doi.org/10.1007/s12035-011-8195-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-011-8195-z