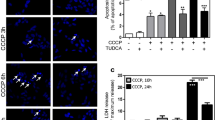
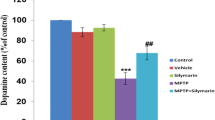
Abstract
Mitochondrial dysfunction and oxidative stress are implicated in the neurodegenerative process in Parkinson’s disease (PD). Moreover, c-Jun N-terminal kinase (JNK) plays an important role in dopaminergic neuronal death in substantia nigra pars compacta. Tauroursodeoxycholic acid (TUDCA) acts as a mitochondrial stabilizer and anti-apoptotic agent in several models of neurodegenerative diseases. Here, we investigated the role of TUDCA in preventing 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurodegeneration in a mouse model of PD. We evaluated whether TUDCA modulates MPTP-induced degeneration of dopaminergic neurons in the nigrostriatal axis, and if that can be explained by regulation of JNK phosphorylation, reactive oxygen species (ROS) production, glutathione S-transferase (GST) catalytic activation, and Akt signaling, using C57BL/6 glutathione S-transferase pi (GSTP) null mice. TUDCA efficiently protected against MPTP-induced dopaminergic degeneration. We have previously demonstrated that exacerbated JNK activation in GSTP null mice resulted in increased susceptibility to MPTP neurotoxicity. Interestingly, pre-treatment with TUDCA prevented MPTP-induced JNK phosphorylation in mouse midbrain and striatum. Moreover, the anti-oxidative role of TUDCA was demonstrated in vivo by impairment of ROS production in the presence of MPTP. Finally, results herein suggest that the survival pathway activated by TUDCA involves Akt signaling, including downstream Bad phosphorylation and NF-κB activation. We conclude that TUDCA is neuroprotective in an in vivo model of PD, acting mainly by modulation of JNK activity and cellular redox thresholds, together with activation of the Akt pro-survival pathway. These results open new perspectives for the pharmacological use of TUDCA, as a modulator of neurodegeneration in PD.








Similar content being viewed by others
References
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
Jackson-Lewis V, Blesa J, Przedborski S (2012) Animal models of Parkinson’s disease. Parkinsonism Relat Disord 18(Suppl 1):S183–S185
Dawson TM, Dawson VL (2003) Molecular pathways of neurodegeneration in Parkinson’s disease. Science 302(5646):819–822
Mosley RL, Benner EJ, Kadiu I, Thomas M, Boska MD, Hasan K, Laurie C, Gendelman HE (2006) Neuroinflammation, oxidative stress and the pathogenesis of Parkinson’s disease. Clin Neurosci Res 6(5):261–281
Vila M, Ramonet D, Perier C (2008) Mitochondrial alterations in Parkinson’s disease: new clues. J Neurochem 107(2):317–328
Miller RL, James-Kracke M, Sun GY, Sun AY (2009) Oxidative and inflammatory pathways in Parkinson’s disease. Neurochem Res 34(1):55–65
Chan P, DeLanney LE, Irwin I, Langston JW, Di Monte D (1991) Rapid ATP loss caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mouse brain. J Neurochem 57:348–351
Nicotra A, Parvez S (2002) Apoptotic molecules and MPTP-induced cell death. Neurotoxicol Teratol 24(5):599–605
Kuan CY, Burke RE (2005) Targeting the JNK signaling pathway for stroke and Parkinson’s diseases therapy. Curr Drug Targets CNS Neurol Disord 4(1):63–67
Peng J, Andersen JK (2003) The role of c-Jun N-terminal kinase (JNK) in Parkinson’s disease. IUBMB Life 55(4-5):267–271
Hunot S, Vila M, Teismann P, Davis RJ, Hirsch EC, Przedborski S, Rakic P, Flavell RA (2004) JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA 101(2):665–670
Nishi K (1997) Expression of c-Jun in dopaminergic neurons of the substantia nigra in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Brain Res 771(1):133–141
Saporito MS, Thomas BA, Scott RW (2000) MPTP activates c-Jun NH(2)-terminal kinase (JNK) and its upstream regulatory kinase MKK4 in nigrostriatal neurons in vivo. J Neurochem 75(3):1200–1208
Castro-Caldas M, Neves Carvalho A, Rodrigues E, Henderson C, Wolf CR, Gama MJ (2012) Glutathione S-transferase pi mediates MPTP-induced c-Jun N-terminal kinase activation in the nigrostriatal pathway. Mol Neurobiol 45(3):466–477
Wang W, Shi L, Xie Y, Ma C, Li W, Su X, Huang S, Chen R, Zhu Z, Mao Z, Han Y, Li M (2004) SP600125, a new JNK inhibitor, protects dopaminergic neurons in the MPTP model of Parkinson’s disease. Neurosci Res 48(2):195–202
Pan J, Qian J, Zhang Y, Ma J, Wang G, Xiao Q, Chen S, Ding J (2010) Small peptide inhibitor of JNKs protects against MPTP-induced nigral dopaminergic injury via inhibiting the JNK-signaling pathway. Lab Invest 90(2):156–167
Lazaridis KN, Gores GJ, Lindor KD (2001) Ursodeoxycholic acid ‘mechanisms of action and clinical use in hepatobiliary disorders’. J Hepatol 35(1):134–146
Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ (1998) Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med 4(3):165–178
Ramalho RM, Ribeiro PS, Solá S, Castro RE, Steer CJ, Rodrigues CM (2004) Inhibition of the E2F-1/p53/Bax pathway by tauroursodeoxycholic acid in amyloid beta-peptide-induced apoptosis of PC12 cells. J Neurochem 90(3):567–575
Viana RJ, Steer CJ, Rodrigues CM (2011) Amyloid-β peptide-induced secretion of endoplasmic reticulum chaperone glycoprotein GRP94. J Alzheimers Dis 27(1):61–73
Viana RJ, Ramalho RM, Nunes AF, Steer CJ, Rodrigues CM (2010) Modulation of amyloid-β peptide-induced toxicity through inhibition of JNK nuclear localization and caspase-2 activation. J Alzheimers Dis 22(2):557–568
Rodrigues CM, Fan G, Ma X, Kren BT, Steer CJ (1998) A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest 101(12):2790–2799
Rodrigues CM, Ma X, Linehan-Stieers C, Fan G, Kren BT, Steer CJ (1999) Ursodeoxycholic acid prevents cytochrome c release in apoptosis by inhibiting mitochondrial membrane depolarization and channel formation. Cell Death Differ 6(9):842–854
Keene CD, Rodrigues CM, Eich T, Linehan-Stieers C, Abt A, Kren BT, Steer CJ, Low WC (2001) A bile acid protects against motor and cognitive deficits and reduces striatal degeneration in the 3-nitropropionic acid model of Huntington’s disease. Exp Neurol 171(2):351–360
Keene CD, Rodrigues CM, Eich T, Chhabra MS, Steer CJ, Low WC (2002) Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc Natl Acad Sci USA 99(16):10671–10676
Solá S, Castro RE, Laires PA, Steer CJ, Rodrigues CM (2003) Tauroursodeoxycholic acid prevents amyloid-beta peptide-induced neuronal death via a phosphatidylinositol 3-kinase-dependent signaling pathway. Mol Med 9(9-12):226–234
Ramalho RM, Borralho PM, Castro RE, Solá S, Steer CJ, Rodrigues CM (2006) Tauroursodeoxycholic acid modulates p53-mediated apoptosis in Alzheimer’s disease mutant neuroblastoma cells. J Neurochem 98(5):1610–1618
Solá S, Amaral JD, Borralho PM, Ramalho RM, Castro RE, Aranha MM, Steer CJ, Rodrigues CM (2006) Functional modulation of nuclear steroid receptors by tauroursodeoxycholic acid reduces amyloid beta-peptide-induced apoptosis. Mol Endocrinol 20(10):2292–2303
Viana RJ, Nunes AF, Castro RE, Ramalho RM, Meyerson J, Fossati S, Ghiso J, Rostagno A, Rodrigues CM (2009) Tauroursodeoxycholic acid prevents E22Q Alzheimer’s Abeta toxicity in human cerebral endothelial cells. Cell Mol Life Sci 66(6):1094–1104
Nunes AF, Amaral JD, Lo AC, Fonseca MB, Viana RJ, Callaerts-Vegh Z, D’Hooge R, Rodrigues CM (2012) TUDCA, a bile acid, attenuates amyloid precursor protein processing and amyloid-beta deposition in APP/PS1 mice. Mol Neurobiol 45(3):440–454
Rodrigues CM, Spellman SR, Solá S, Grande AW, Linehan-Stieers C, Low WC, Steer CJ (2002) Neuroprotection by a bile acid in an acute stroke model in the rat. J Cereb Blood Flow Metab 22(4):463–471
Rodrigues CM, Sola S, Nan Z, Castro RE, Ribeiro PS, Low WC, Steer CJ (2003) Tauroursodeoxycholic acid reduces apoptosis and protects against neurological injury after acute hemorrhagic stroke in rats. Proc Natl Acad Sci USA 100(10):6087–6092
Duan WM, Rodrigues CM, Zhao LR, Steer CJ, Low WC (2002) Tauroursodeoxycholic acid improves the survival and function of nigral transplants in a rat model of Parkinson’s disease. Cell Transplant 11(3):195–205
Ved R, Saha S, Westlund B, Perier C, Burnam L, Sluder A, Hoener M, Rodrigues CM, Alfonso A, Steer C, Liu L, Przedborski S, Wolozin B (2005) Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J Biol Chem 280(52):42655–42668
Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR (1998) Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc Natl Acad Sci USA 95(9):5275–5280
Castro-Caldas M, Neves Carvalho A, Peixeiro I, Rodrigues E, Lechner MC, Gama MJ (2009) GSTpi expression in MPTP-induced dopaminergic neurodegeneration of C57BL/6 mouse midbrain and striatum. J Mol Neurosci 38(2):114–127
Kong XC, Zhang D, Qian C, Liu GT, Bao XQ (2011) FLZ, a novel HSP27 and HSP70 inducer, protects SH-SY5Y cells from apoptosis caused by MPP(+). Brain Res 1383:99–107
Latchman DS (2005) HSP27 and cell survival in neurones. Int J Hyperth 21(5):393–402
Castro RE, Solá S, Ramalho RM, Steer CJ, Rodrigues CM (2004) The bile acid tauroursodeoxycholic acid modulates phosphorylation and translocation of bad via phosphatidylinositol 3-kinase in glutamate-induced apoptosis of rat cortical neurons. J Pharmacol Exp Ther 311(2):845–852
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129(7):1261–1274
Romashkova JA, Makarov SS (1999) NF-kappaB is a target of AKT in anti-apoptotic PDGF signaling. Nature 401(6748):86–90
Kane LP, Shapiro VS, Stokoe D, Weiss A (1999) Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol 9(11):601–604
Menegon A, Board PG, Blackburn AC, Mellick GD, Le Couteur DG (1998) Parkinson’s disease, pesticides, and glutathione transferase polymorphisms. Lancet 352(9137):1344–1346
Vilar R, Coelho H, Rodrigues E, Gama MJ, Rivera I, Taioli E, Lechner MC (2007) Association of A313 G polymorphism (GSTP1*B) in the glutathione-S-transferase P1 gene with sporadic Parkinson’s disease. Eur J Neurol 14(2):156–161
Tsang AH, Chung KK (2009) Oxidative and nitrosative stress in Parkinson’s disease. Biochim Biophys Acta 1792(7):643–650
Stetler RA, Cao G, Gao Y, Zhang F, Wang S, Weng Z, Vosler P, Zhang L, Signore A, Graham SH, Chen J (2008) Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci 28(49):13038–13055
Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, Davis RJ, Ronai Z (1999) Regulation of JNK signaling by GSTp. EMBO J 18(5):1321–1334
Elsby R, Kitteringham NR, Goldring CE, Lovatt CA, Chamberlain M, Henderson CJ, Wolf CR, Park BK (2003) Increased constitutive c-Jun N-terminal kinase signaling in mice lacking glutathione S-transferase Pi. J Biol Chem 278(25):22243–22249
Yan LJ, Rajasekaran NS, Sathyanarayanan S, Benjamin IJ (2005) Mouse HSF1 disruption perturbs redox state and increases mitochondrial oxidative stress in kidney. Antioxid Redox Signal 7(3-4):465–471
Kitani K, Kanai S, Sato Y, Ohta M, Nokubo M (1994) Ursodeoxycholic acid reduces the systemic toxicity of 1,2-dichloro,4-nitrobenzene by stimulating hepatic glutathione S-transferase in mice. Life Sci 54(14):983–989
Wang XT, Pei DS, Xu J, Guan QH, Sun YF, Liu XM, Zhang GY (2007) Opposing effects of Bad phosphorylation at two distinct sites by Akt1 and JNK1/2 on ischemic brain injury. Cell Signal 19(9):1844–1856
del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G (1997) Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278:687–689
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241
Parry GJ, Rodrigues CM, Aranha MM, Hilbert SJ, Davey C, Kelkar P, Low WC, Steer CJ (2010) Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic acid in patients with amyotrophic lateral sclerosis. Clin Neuropharmacol 33(1):17–21
Morrish PK, Rakshi JS, Bailey DL, Sawle GV, Brooks DJ (1998) Measuring the rate of progression and estimating the preclinical period of Parkinson’s disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry 64(3):314–319
Acknowledgments
Supported by Fundação para a Ciência e a Tecnologia (FCT) and FEDER through grants PPCDT/SAU-FCF/58171/2004 and PEst-OE/SAU/UI4013/2011, and Ph.D. fellowship SFRH/BD/39897/2007 (to ANC).
Statement of Conflicts of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Castro-Caldas and A. Neves Carvalho are joint first authors.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 15 kb)
Rights and permissions
About this article
Cite this article
Castro-Caldas, M., Carvalho, A.N., Rodrigues, E. et al. Tauroursodeoxycholic Acid Prevents MPTP-Induced Dopaminergic Cell Death in a Mouse Model of Parkinson’s Disease. Mol Neurobiol 46, 475–486 (2012). https://doi.org/10.1007/s12035-012-8295-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-012-8295-4