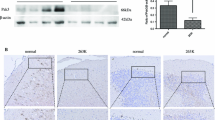
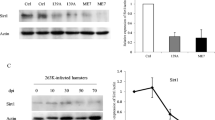
Abstract
Polo-like kinases (PLKs) consist of a family of kinases which play critical roles during multiple stages of cell cycle progression. Increase of PLK1 and decrease of PLK3 are associated with the developments and metastases of many types of human malignant tumors; however, the situations of PLKs in prion diseases are less understood. Using Western blots and immunohistochemical and immunofluorescent assays, marked increase of PLK1 and decrease of PLK3 were observed in the brains of scrapie strain 263K-infected hamsters, presenting obviously a time-dependent phenomenon along with disease progression. Similar alterations of PLKs were also detected in a scrapie infectious cell line SMB-S15. Both PLK1 and PLK3 were observed in neurons by confocal microscopy. Accompanying with the changes of PLKs in the brains of 263K-infected hamsters, Cdc25C and its phosphorylated forms (p-Cdc25C-Ser198 and p-Cdc25C-Ser216) were significantly down-regulated, whereas Cyclin B1 and PCNA were obviously up-regulated, while phospho-histone H3 remained almost unchanged. Moreover, exposure of the cytotoxic peptide PrP106-126 on the primary cultured cortical neuron cells induced similar changes of cellular PLKs and some cell cycle-related proteins, such as Cdc25C and its phosphorylated forms, phospho-histone H3. Those results illustrate obviously aberrant expressions of cell cycle regulatory proteins in the prion-infected neurons, which may lead to the cell cycle arrest at M phase. Possibly due to the ill-regulation of some key cell cycle events during prion infection, together with the fact that neurons are unable to complete mitosis, the cell cycle reentry in prion-infected neurons is definitely abortive, which may lead to neuron apoptosis and neuron degeneration.









Similar content being viewed by others
References
Castilla J, Saa P, Hetz C, Soto C (2005) In vitro generation of infectious scrapie prions. Cell 121(2):195–206. doi:10.1016/j.cell.2005.02.011
Legname G, Baskakov IV, Nguyen HO, Riesner D, Cohen FE, DeArmond SJ, Prusiner SB (2004) Synthetic mammalian prions. Science 305(5684):673–676. doi:10.1126/science.1100195
Lopes JP, Oliveira CR, Agostinho P (2009) Cell cycle re-entry in Alzheimer's disease: a major neuropathological characteristic? Curr Alzheimer Res 6(3):205–212
Petersen RC (2000) Mild cognitive impairment: transition between aging and Alzheimer's disease. Neurologia 15(3):93–101
van de Weerdt BC, Medema RH (2006) Polo-like kinases: a team in control of the division. Cell Cycle 5(8):853–864
Lane HA, Nigg EA (1996) Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol 135(6 Pt 2):1701–1713
Abrieu A, Brassac T, Galas S, Fisher D, Labbe JC, Doree M (1998) The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J Cell Sci 111(Pt 12):1751–1757
Sumara I, Gimenez-Abian JF, Gerlich D, Hirota T, Kraft C, de la Torre C, Ellenberg J, Peters JM (2004) Roles of polo-like kinase 1 in the assembly of functional mitotic spindles. Curr Biol 14(19):1712–1722. doi:10.1016/j.cub.2004.09.049
Neef R, Preisinger C, Sutcliffe J, Kopajtich R, Nigg EA, Mayer TU, Barr FA (2003) Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J Cell Biol 162(5):863–875. doi:10.1083/jcb.200306009
Toyoshima-Morimoto F, Taniguchi E, Nishida E (2002) Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep 3(4):341–348. doi:10.1093/embo-reports/kvf069
Toyoshima-Morimoto F, Taniguchi E, Shinya N, Iwamatsu A, Nishida E (2001) Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature 410(6825):215–220. doi:10.1038/35065617
Donohue PJ, Alberts GF, Guo Y, Winkles JA (1995) Identification by targeted differential display of an immediate early gene encoding a putative serine/threonine kinase. J Biol Chem 270(17):10351–10357
Zimmerman WC, Erikson RL (2007) Finding Plk3. Cell Cycle 6(11):1314–1318
Seeburg DP, Pak D, Sheng M (2005) Polo-like kinases in the nervous system. Oncogene 24(2):292–298. doi:10.1038/sj.onc.1208277
Yang Y, Bai J, Shen R, Brown SAN, Komissarova E, Huang Y, Jiang N, Alberts GF, Costa M, Lu L, Winkles JA, Dai W (2008) Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1 alpha under hypoxic conditions. Cancer Research 68(11):4077–4085. doi:10.1158/0008-5472.can-07-6182
Luo J, Liu X (2012) Polo-like kinase 1, on the rise from cell cycle regulation to prostate cancer development. Protein & Cell 3(3):182–197. doi:10.1007/s13238-012-2020-y
Yao HL, Han J, Gao JM, Zhang J, Zhang BY, Guo YJ, Nie K, Gao C, Wang XF, Dong XP (2005) Comparative study of the effects of several chemical and physical treatments on the activity of protease resistance and infectivity of scrapie strain 263K. J Vet Med B Infect Dis Vet Public Health 52(10):437–443. doi:10.1111/j.1439-0450.2005.00897.x
Shi Q, Zhang BY, Gao C, Zhang J, Jiang HY, Chen C, Han J, Dong XP (2012) Mouse-adapted scrapie strains 139A and ME7 overcome species barrier to induce experimental scrapie in hamsters and changed their pathogenic features. Virol J 9:63. doi:10.1186/1743-422X-9-63
Gao JM, Gao C, Han J, Zhou XB, Xiao XL, Zhang J, Chen L, Zhang BY, Hong T, Dong XP (2004) Dynamic analyses of PrP and PrP(Sc) in brain tissues of golden hamsters infected with scrapie strain 263K revealed various PrP forms. Biomed Environ Sci 17(1):8–20
Zhang J, Chen L, Zhang BY, Han J, Xiao XL, Tian HY, Li BL, Gao C, Gao JM, Zhou XB, Ma GP, Liu Y, Xu CM, Dong XP (2004) Comparison study on clinical and neuropathological characteristics of hamsters inoculated with scrapie strain 263K in different challenging pathways. Biomed Environ Sci 17(1):65–78
Hilgenberg LG, Smith MA (2007) Preparation of dissociated mouse cortical neuron cultures. J Vis Exp (10):562. doi:10.3791/562
Xie S, Xie B, Lee MY, Dai W (2005) Regulation of cell cycle checkpoints by polo-like kinases. Oncogene 24(2):277–286. doi:10.1038/sj.onc.1208218
Myer DL, Bahassi EM, Stambrook PJ (2005) The Plk3–Cdc25 circuit. Oncogene 24(2):299–305. doi:10.1038/sj.onc.1208278
Wei Y, Mizzen CA, Cook RG, Gorovsky MA, Allis CD (1998) Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc Natl Acad Sci U S A 95(13):7480–7484
Galand P, Degraef C (1989) Cyclin/PCNA immunostaining as an alternative to tritiated thymidine pulse labelling for marking S phase cells in paraffin sections from animal and human tissues. Cell Tissue Kinet 22(5):383–392
Forloni G, Angeretti N, Chiesa R, Monzani E, Salmona M, Bugiani O, Tagliavini F (1993) Neurotoxicity of a prion protein fragment. Nature 362(6420):543–546. doi:10.1038/362543a0
Ettaiche M, Pichot R, Vincent JP, Chabry J (2000) In vivo cytotoxicity of the prion protein fragment 106–126. J Biol Chem 275(47):36487–36490. doi:10.1074/jbc.C000579200
Glover DM, Hagan IM, Tavares AA (1998) Polo-like kinases: a team that plays throughout mitosis. Genes Dev 12(24):3777–3787
de Cárcer G, Manning G, Malumbres M (2011) From Plk1 to Plk5: functional evolution of polo-like kinases. Cell Cycle 10(14):2255–2262. doi:10.4161/cc.10.14.16494
Eckerdt F, Yuan J, Strebhardt K (2005) Polo-like kinases and oncogenesis. Oncogene 24(2):267–276. doi:10.1038/sj.onc.1208273
Wang Z-X, Xue D, Liu Z-L, Lu B-B, Bian H-B, Pan X, Yin Y-M (2012) Overexpression of polo-like kinase 1 and its clinical significance in human non-small cell lung cancer. The International Journal of Biochemistry & Cell Biology 44(1):200–210. doi:10.1016/j.biocel.2011.10.017
Yang Y, Bai J, Shen R, Brown SA, Komissarova E, Huang Y, Jiang N, Alberts GF, Costa M, Lu L, Winkles JA, Dai W (2008) Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1 alpha under hypoxic conditions. Cancer Res 68(11):4077–4085. doi:10.1158/0008-5472.CAN-07-6182
Song B, Davis K, Liu XS, Lee HG, Smith M, Liu X (2011) Inhibition of Polo-like kinase 1 reduces beta-amyloid-induced neuronal cell death in Alzheimer's disease. Aging (Albany NY) 3(9):846–851
Harris PL, Zhu X, Pamies C, Rottkamp CA, Ghanbari HA, McShea A, Feng Y, Ferris DK, Smith MA (2000) Neuronal polo-like kinase in Alzheimer disease indicates cell cycle changes. Neurobiol Aging 21(6):837–841
Webber KM, Raina AK, Marlatt MW, Zhu X, Prat MI, Morelli L, Casadesus G, Perry G, Smith MA (2005) The cell cycle in Alzheimer disease: a unique target for neuropharmacology. Mech Ageing Dev 126(10):1019–1025. doi:10.1016/j.mad.2005.03.024
Martin SA, Ouchi T (2008) Cellular commitment to reentry into the cell cycle after stalled DNA is determined by site-specific phosphorylation of Chk1 and PTEN. Mol Cancer Ther 7(8):2509–2516. doi:10.1158/1535-7163.MCT-08-0199
Lopes JP, Oliveira CR, Agostinho P (2009) Cdk5 acts as a mediator of neuronal cell cycle re-entry triggered by amyloid-beta and prion peptides. Cell Cycle 8(1):97–104
Zhang J, Li H, Herrup K (2010) Cdk5 nuclear localization is p27-dependent in nerve cells: implications for cell cycle suppression and caspase-3 activation. Journal of Biological Chemistry 285(18):14052–14061. doi:10.1074/jbc.M109.068262
Chang K-H, de Pablo Y, H-p L, H-g L, Smith MA, Shah K (2010) Cdk5 is a major regulator of p38 cascade: relevance to neurotoxicity in Alzheimer’s disease. Journal of Neurochemistry. doi:10.1111/j.1471-4159.2010.06687.x
Dobashi Y, Kudoh T, Matsumine A, Toyoshima K, Akiyama T (1995) Constitutive overexpression of CDK2 inhibits neuronal differentiation of rat pheochromocytoma PC12 cells. J Biol Chem 270(39):23031–23037
Wang Z, Trope CG, Florenes VA, Suo Z, Nesland JM, Holm R (2010) Overexpression of CDC25B, CDC25C and phospho-CDC25C (Ser216) in vulvar squamous cell carcinomas are associated with malignant features and aggressive cancer phenotypes. BMC Cancer 10:233. doi:10.1186/1471-2407-10-233
Ouyang B, Li W, Pan H, Meadows J, Hoffmann I, Dai W (1999) The physical association and phosphorylation of Cdc25C protein phosphatase by Prk. Oncogene 18(44):6029–6036. doi:10.1038/sj.onc.1202983
el Bahassi M, Hennigan RF, Myer DL, Stambrook PJ (2004) Cdc25C phosphorylation on serine 191 by Plk3 promotes its nuclear translocation. Oncogene 23(15):2658–2663. doi:10.1038/sj.onc.1207425
Tang L, Wang TT, Wu YT, Zhou CY, Huang HF (2009) High expression levels of cyclin B1 and Polo-like kinase 1 in ectopic endometrial cells associated with abnormal cell cycle regulation of endometriosis. Fertil Steril 91(4):979–987. doi:10.1016/j.fertnstert.2008.01.041
Stukenberg PT, Lustig KD, McGarry TJ, King RW, Kuang J, Kirschner MW (1997) Systematic identification of mitotic phosphoproteins. Curr Biol 7(5):338–348
Matsumoto-Taniura N, Pirollet F, Monroe R, Gerace L, Westendorf JM (1996) Identification of novel M phase phosphoproteins by expression cloning. Mol Biol Cell 7(9):1455–1469
Yaffe MP (1997) Mitochondrial morphogenesis: fusion factor for fly fertility. Curr Biol 7(12):R782–783
Soria JC, Jang SJ, Khuri FR, Hassan K, Liu D, Hong WK, Mao L (2000) Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res 60(15):4000–4004
Choi JA, Kim JY, Lee JY, Kang CM, Kwon HJ, Yoo YD, Kim TW, Lee YS, Lee SJ (2001) Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int J Oncol 19(4):837–844
Vincent I, Jicha G, Rosado M, Dickson DW (1997) Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's disease brain. J Neurosci 17(10):3588–3598
Klein JA, Ackerman SL (2003) Oxidative stress, cell cycle, and neurodegeneration. J Clin Invest 111(6):785–793. doi:10.1172/JCI18182
Yang Y, Geldmacher DS, Herrup K (2001) DNA replication precedes neuronal cell death in Alzheimer's disease. J Neurosci 21(8):2661–2668
Krantic S, Mechawar N, Reix S, Quirion R (2005) Molecular basis of programmed cell death involved in neurodegeneration. Trends Neurosci 28(12):670–676. doi:10.1016/j.tins.2005.09.011
Wang GR, Shi S, Gao C, Zhang BY, Tian C, Dong CF, Zhou RM, Li XL, Chen C, Han J, Dong XP (2010) Changes of tau profiles in brains of the hamsters infected with scrapie strains 263 K or 139 A possibly associated with the alteration of phosphate kinases. BMC Infect Dis 10:86. doi:10.1186/1471-2334-10-86
Satoh J, Obayashi S, Misawa T, Sumiyoshi K, Oosumi K, Tabunoki H (2009) Protein microarray analysis identifies human cellular prion protein interactors. Neuropathology and Applied Neurobiology 35(1):16–35. doi:10.1111/j.1365-2990.2008.00947.x
Busser J, Geldmacher DS, Herrup K (1998) Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer's disease brain. J Neurosci 18(8):2801–2807
Nagy Z, Esiri MM, Smith AD (1997) Expression of cell division markers in the hippocampus in Alzheimer's disease and other neurodegenerative conditions. Acta Neuropathol 93(3):294–300
H-g L, Casadesus G, Nunomura A, Zhu X, Castellani RJ, Richardson SL, Perry G, Felsher DW, Petersen RB, Smith MA (2009) The neuronal expression of MYC causes a neurodegenerative phenotype in a novel transgenic mouse. The American Journal of Pathology 174(3):891–897. doi:10.2353/ajpath.2009.080583
Acknowledgments
This work was supported by the Chinese National Natural Science Foundation Grant (81101302, 81273202), China Mega-Project for Infectious Disease (2011ZX10004-101, 2012ZX10004215), SKLID Development Grant (2012SKLID102), and Sci-tech Innovation Team of Jiangsu University (2008-018-02). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hui Wang and Chan Tian contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
NeuN-specific IFA of the primary cultured rat cortical neurons. The images of NeuN (red), DAPI (blue) and merge are indicated above (PDF 42 kb)
Supplemental Fig. 2
Western blots of Cyclin the lysates of the cell line SMB-S15 and SMB-PS (left). Quantitative analyses of the gray numerical values of the blots vs. that of the individual β-actin are showed on the right. The average relative gray value is calculated from three independent blots and presented as mean ± SEM. **p<0.001 significant compared to controls are illustrated on the top (PDF 106 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Tian, C., Xu, Y. et al. Abortive Cell Cycle Events in the Brains of Scrapie-Infected Hamsters with Remarkable Decreases of PLK3/Cdc25C and Increases of PLK1/Cyclin B1. Mol Neurobiol 48, 655–668 (2013). https://doi.org/10.1007/s12035-013-8455-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8455-1