Abstract
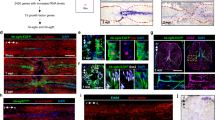
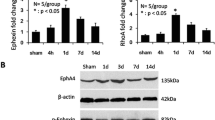
High mobility group box 1 (HMGB1, also called amphoterin) facilitates neurite outgrowth in early development, yet can exacerbate pathology and inhibit regeneration by inducing adverse neuroinflammation when released from dying cells, suggesting that HMGB1 plays a critical, yet undefined role in neuroregeneration. We explored whether HMGB1 contributes to recovery after complete spinal cord transection in adult zebrafish. Quantitative PCR and in situ hybridization revealed that HMGB1 mRNA levels decreased between 12 h to 11 days after spinal cord injury (SCI), then returned to basal levels by 21 days. Western blot and immunohistological analyses indicated that the time course of HMGB1 protein expression after SCI parallels that of mRNA. Immunofluorescence staining revealed that HMGB1 translocates from nuclei into the cytoplasm of spinal motoneurons at 4 and 12 h (acute stage) following SCI, then accumulates in the nuclei of motoneurons during the ensuing chronic stage (after 6 days following SCI). Immunohistology of transgenic zebrafish, expressing green fluorescent protein in blood vessels, showed enhanced HMGB1 expression in blood vessels in the vicinity of motoneurons. Application of anti-sense HMGB1 morpholinos inhibited locomotor recovery by 34 % and decreased axonal regeneration by 34 % compared to fish treated with a control morpholino. The present study shows that HMGB1 expression increases in both endothelial cells and motoneurons, suggesting that HMGB1 promotes recovery from SCI not only through enhancing neuroregeneration, but also by increasing angiogenesis. The inflammatory effects of HMGB1 are minimized through the decrease in HMGB1 expression during the acute stage.






Similar content being viewed by others
References
Ma L, Yu YM, Guo Y, Hart RP, Schachner M (2012) Cysteine- and glycine-rich protein 1a is involved in spinal cord regeneration in adult zebrafish. Eur J Neurosci 35:353–365
Fang P, Schachner M, Shen YQ (2012) HMGB1 in development and diseases of the central nervous system. Mol Neurobiol 45:499–506
Guazzi S, Strangio A, Franzi AT, Bianchi ME (2003) HMGB1, an architectural chromatin protein and extracellular signalling factor, has a spatially and temporally restricted expression pattern in mouse brain. Gene Expr Patterns 3:29–33
Enokido Y, Yoshitake A, Ito H, Okazawa H (2008) Age-dependent change of HMGB1 and DNA double-strand break accumulation in mouse brain. Biochem Biophys Res Commun 376:128–133
Daston MM, Ratner N (1994) Amphoterin (P30, HMG-1) and RIP are early markers of oligodendrocytes in the developing rat spinal cord. J Neurocytol 23:323–332
Tenenbaum T, Essmann F, Adam R, Seibt A, Janicke RU, Novotny GE, Galla HJ, Schroten H (2006) Cell death, caspase activation, and HMGB1 release of porcine choroid plexus epithelial cells during Streptococcus suis infection in vitro. Brain Res 1100:1–12
Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS (2011) HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci 31:1081–1092
Zhao X, Kuja-Panula J, Rouhiainen A, Chen YC, Panula P, Rauvala H (2011) High mobility group box-1 (HMGB1; amphoterin) is required for zebrafish brain development. J Biol Chem 286:23200–23213
Chou DK, Evans JE, Jungalwala FB (2001) Identity of nuclear high-mobility-group protein, HMG-1, and sulfoglucuronyl carbohydrate-binding protein, SBP-1, in brain. J Neurochem 77:120–131
Merenmies J, Pihlaskari R, Laitinen J, Wartiovaara J, Rauvala H (1991) 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J Biol Chem 266:16722–16729
Rauvala H, Pihlaskari R (1987) Isolation and some characteristics of an adhesive factor of brain that enhances neurite outgrowth in central neurons. J Biol Chem 262:16625–16635
Kawabata H, Setoguchi T, Yone K, Souda M, Yoshida H, Kawahara K, Maruyama I, Komiya S (2010) High mobility group box 1 is upregulated after spinal cord injury and is associated with neuronal cell apoptosis. Spine 35:1109–1115 (Phila Pa 1976)
Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK (2006) HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci 26:6413–6421
Blanco P, Palucka AK, Pascual V, Banchereau J (2008) Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev 19:41–52
Rauvala H, Rouhiainen A (2010) Physiological and pathophysiological outcomes of the interactions of HMGB1 with cell surface receptors. Biochim Biophys Acta 1799:164–170
Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C et al (2007) Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 8:487–496
Urbonaviciute V, Furnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA et al (2008) Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med 205:3007–3018
Wahamaa H, Schierbeck H, Hreggvidsdottir HS, Palmblad K, Aveberger AC, Andersson U, Harris HE (2011) High mobility group box protein 1 in complex with lipopolysaccharide or IL-1 promotes an increased inflammatory phenotype in synovial fibroblasts. Arthritis Res Ther 13:R136
Huang Y, Xie K, Li J, Xu N, Gong G, Wang G, Yu Y, Dong H, Xiong L (2011) Beneficial effects of hydrogen gas against spinal cord ischemia-reperfusion injury in rabbits. Brain Res 10:125–136
Wang Q, Ding Q, Zhou Y, Gou X, Hou L, Chen S, Zhu Z, Xiong L (2009) Ethyl pyruvate attenuates spinal cord ischemic injury with a wide therapeutic window through inhibiting high-mobility group box 1 release in rabbits. Anesthesiology 110:1279–1286
Shibasaki M, Sasaki M, Miura M, Mizukoshi K, Ueno H, Hashimoto S, Tanaka Y, Amaya F (2010) Induction of high mobility group box-1 in dorsal root ganglion contributes to pain hypersensitivity after peripheral nerve injury. Pain 149:514–521
Hayakawa K, Pham LD, Katusic ZS, Arai K, Lo EH (2012) Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci U S A 109:7505–7510
Lawson ND, Weinstein BM (2002) In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 248:307–318
Fang P, Lin JF, Pan HC, Shen YQ, Schachner M (2012) A surgery protocol for adult zebrafish spinal cord injury. J Genet Genomics 39:481–487
Goff LA, Bowers J, Schwalm J, Howerton K, Getts RC, Hart RP (2004) Evaluation of sense-strand mRNA amplification by comparative quantitative PCR. BMC Genomics 5:76
Song G, Li Q, Long Y, Hackett PB, Cui Z (2012) Effective expression-independent gene trapping and mutagenesis mediated by Sleeping Beauty transposon. J Genet Genomics 39:503–520
Pei DS, Sun YH, Chen SP, Wang YP, Hu W, Zhu ZY (2007) Zebrafish GAPDH can be used as a reference gene for expression analysis in cross-subfamily cloned embryos. Analytical biochemistry 363:291–293
Bernhardt RR, Tongiorgi E, Anzini P, Schachner M (1996) Increased expression of specific recognition molecules by retinal ganglion cells and by optic pathway glia accompanies the successful regeneration of retinal axons in adult zebrafish. J Comp Neurol 376:253–264
Lin JF, Pan HC, Ma LP, Shen YQ, Schachner M (2012) The Cell Neural Adhesion Molecule Contactin-2 (TAG-1) is beneficial for functional recovery after spinal cord injury in adult zebrafish. PLoS One 7:e52376
Guo Y, Ma L, Cristofanilli M, Hart RP, Hao A, Schachner M (2011) Transcription factor Sox11b is involved in spinal cord regeneration in adult zebrafish. Neuroscience 172:329–341
Pan HC, Lin JF, Ma LP, Shen YQ, Schachner M (2013) Major vault protein promotes locomotor recovery and regeneration after spinal cord injury in adult zebrafish. Eur J Neurosci 37:203–211
Nasevicius A, Ekker SC (2000) Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet 26:216–220
Becker CG, Lieberoth BC, Morellini F, Feldner J, Becker T, Schachner M (2004) L1.1 is involved in spinal cord regeneration in adult zebrafish. J Neurosci 24:7837–7842
Becker T, Wullimann MF, Becker CG, Bernhardt RR, Schachner M (1997) Axonal regrowth after spinal cord transection in adult zebrafish. J Comp Neurol 377:577–595
Yu YM, Cristofanilli M, Valiveti A, Ma L, Yoo M, Morellini F, Schachner M (2011) The extracellular matrix glycoprotein tenascin-C promotes locomotor recovery after spinal cord injury in adult zebrafish. Neuroscience 183:238–250
Hugnot JP, Franzen R (2011) The spinal cord ependymal region: a stem cell niche in the caudal central nervous system. Front Biosci 16:1044–1059
Becker T, Becker CG (2001) Regenerating descending axons preferentially reroute to the gray matter in the presence of a general macrophage/microglial reaction caudal to a spinal transection in adult zebrafish. J Comp Neurol 433:131–147
Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A et al (2008) The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci 28:12023–12031
Kyritsis N, Kizil C, Zocher S, Kroehne V, Kaslin J, Freudenreich D, Iltzsche A, Brand M (2012) Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 338:1353–1356
Kitahara T, Takeishi Y, Harada M, Niizeki T, Suzuki S, Sasaki T, Ishino M, Bilim O, Nakajima O, Kubota I (2008) High-mobility group box 1 restores cardiac function after myocardial infarction in transgenic mice. Cardiovasc Res 80:40–46
Lei C, Lin S, Zhang C, Tao W, Dong W, Hao Z, Liu M, Wu B (2013) Effects of high-mobility group box1 on cerebral angiogenesis and neurogenesis after intracerebral hemorrhage. Neuroscience 229:12–19
Acknowledgments
This project is supported by the National Natural Science Foundation of China (No. 81072622, No. 31271580), Guangdong Natural Science Foundation (S2012010009480) and the Li Kashing Foundation. Melitta Schachner is New Jersey Professor of Spinal Cord Research. We are grateful to Zilong Wen for kindly providing the Fli1a::EGFP transgenic fish.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 838 kb)
Rights and permissions
About this article
Cite this article
Fang, P., Pan, HC., Lin, S.L. et al. HMGB1 Contributes to Regeneration After Spinal Cord Injury in Adult Zebrafish. Mol Neurobiol 49, 472–483 (2014). https://doi.org/10.1007/s12035-013-8533-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8533-4