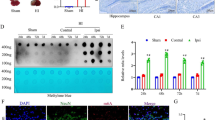
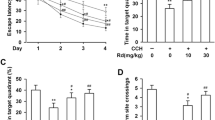
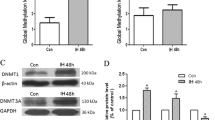
Abstract
Chronic cerebral hypoperfusion is associated with cognitive decline in aging and age-related neurodegenerative disease. Epigenetic mechanisms are involved in the maintenance of long-term hypoxia-adapted cellular phenotypes. In the present study, the epigenetic signatures such as DNA methylation and histone acetylation, as well as S-adenosylmethionine (SAM) cycle using chronic cerebral hypoperfusion rat model were explored. Chronic cerebral hypoxia-induced global DNA hypermethylation associated with the increase of DNA methyltransferase (DNMT) 3A as well as alteration of SAM cycle. Meanwhile, an enhanced level of global histone H4 acetylation accompanied with the upregulation of histone acetyltransferase, p300/CREB-binding protein (CBP), and the downregulation of histone deacetylases (HDACs), was also observed. SAM could improve spatial capacity through the upregulation of acetylcholine and brain-derived neurotrophic factor (BDNF) rather than alteration of DNA methylation levels. In conclusion, we have demonstrated a genome-wide adjustment of DNA methylation and histone acetylation under chronic cerebral hypoxic conditions in a rat’s brain. These epigenetic signatures may represent an additional mechanism to promote and maintain a hypoxic-adapted cellular responds with a potential role in memory deficits.








Similar content being viewed by others
References
Bakker FC, Klijn CJM, Jennekens-Schinkel A, Kappelle LJ (2000) Cognitive disorders in patients with occlusive disease of the carotid artery: a systematic review of the literature. J Neurol 247:669–676
Sekhon LHS, Morgan MK, Spence I, Weber N (1997) Chronic cerebral hypoperfusion: pathological and behavioral consequences. Neurosurgery 40:548–556
Tsai YP, Wu KJ (2013) Epigenetic regulation of hypoxia-responsive gene expression: focusing on chromatin and DNA modifications. Int J Cancer. doi:10.1002/ijc.28190
Watson JA, Watson CJ, McCann A, Baugh J (2010) Epigenetics, the epicenter of the hypoxic response. Epigenetics 16:293–296
Kim SH, Jeong JW, Park JA, Lee JW, Seo JH, Jung BK, Bae MK, Kim KW (2007) Regulation of the HIF-1 alpha stability by histone deacetylases. Oncol Rep 17:647–651
Kenneth NS, Mudie S, van Uden P, Rocha S (2008) SWI/SNF regulates the cellular response to hypoxia. J Biol Chem 284:4123–4131
Fuso A, Seminara L, Cavallaro RA, D'Anselmi F, Scarpa S (2005) S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol Cell Neurosci 28:195–204
Tchantchou F, Shea TB (2008) Folate deprivation, the methionine cycle, and Alzheimer’s disease. Vitam Horm 79:83–97
Quadri P, Fragiacomo C, Pezzati R, Zanda E, Forloni G, Tettamanti M, Lucca U (2004) Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am J Clin Nutr 80:114–122
Chiang PK, Gordon RK, Tal J, Zeng GC, Doctor BP, Pardhasaradhi K, McCann PP (1996) Sadenosylmethionine and methylation. FASEB J 10:471–480
Medina MA, Urdiales JL, Amores-Sanchez MI (2001) Roles of homocysteine in cell metabolism: old and new function. Eur J Biochem 268:3871–3882
Gu X, Sun J, Li S, Wu X, Li L (2013) Oxidative stress induces DNA demethylation and histone acetylation in SH-SY5Y cells: potential epigenetic mechanisms in gene transcription in Aβ production. Neurobiol Aging 34:1069–1079
Guo X, Wu X, Ren L, Liu G, Li L (2011) Epigenetic mechanisms of Aβ production in anisomycin-treated SH-SY5Y cells. Neurosci 194:271–281
Ni J, Ohta H, Matsumoto K, Watanabe H (1994) Progressive cognitive impairment following chronic cerebral hypoperfusion induced by permanent occlusion of bilateral carotid arteries in rats. Brain Res 653:231–236
Sun MK, Xu H, Alkon DL (2002) Pharmacological protection of synaptic function, spatial learning, and memory from transient hypoxia in rats. J Pharmacol Exp Ther 300:408–416
Liu H, Xing A, Wang X, Liu G, Li L (2012) Regulation of β-amyloid level in the brain of rats with cerebrovascular hypoperfusion. Neurobiol Aging 33(826):e31–e42
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates. Academic Press, London
Bhattacharya SK, Ramchandani S, Cervoni N, Szyf M (1999) A mammalian protein with specific demethylase activity for mCpG DNA. Nature 397:579–583
Mesulam MM, Geula C (1988) Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetytransferase. J Comp Neurol 275:216–240
Heimer L (2003) The legacy of the silver methods and the new anatomy of the basal forebrain: implications for neuropsychiatry and drug abuse. Scand J Psychol 44:189–201
Farkas E, Luiten PG, Bari F (2007) Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 54:162–180
de la Torre JC, Stefano GB (2000) Evidence that Alzheimer’s disease is a microvascular disorder: the role of constitutive nitric oxide. Brain Res Rev 34:119–136
Xia X, Lemieux ME, Li W, Carroll JS, Brown M, Liu XS, Kung AL (2009) Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci U S A 106:4260–4265
Tanaka T, Kato H, Kojima I, Onse T, Son D, Tawakami T, Yatagawa T, Inagi R, Fujita T, Nangaku M (2006) Hypoxia and expression of hypoxia-inducible factor in the aging kidney. J Gerontol A Biol Sci Med Sci 61:795–805
Aalami O, Fang TD, Song HM, Nacamuli RP (2003) Physiological features of aging persons. Arch Surg 138:1068–1076
Shahrzad S, Bertrand K, Minhas K, Coomber BL (2007) Induction of DNA hypomethylation by tumor hypoxia. Epigenetics 2:119–125
Watson JA, Watson CJ, McCrohan AM, Woodfine K, Tosetto M, McDaid J, Gallagher E, Betts D, Baugh J, O'Sullivan J, Murrell A, Watson RW, McCann A (2009) Generation of an epigenetic signature by chronic hypoxia in prostate cells. Hum Mol Genet 18:3594–3604
Hermann A, Gowher H, Jeltch A (2004) Biochemistry and biology of mammalian DNA methyltransferases. Cell Mol Life Sci 61:2571–2587
Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C, Volinia S, Guler G, Morrison CD, Chan KK, Marcucci G, Calin GA, Huebner K, Croce CM (2007) MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A 104:15805–15810
van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN (2008) Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A 105:13027–13032
Tohgi H, Utsugisawa K, Nagane Y, Yoshimura M, Genda Y, Ukitsu M (1999) Reduction with age in methylcytosine in the promoter region −224 approximately −101 of the amyloid precursor protein gene in autopsy human cortex. Brain Res Mol Brain Res 70:288–292
Fuso A, Nicolia V, Cavallaro RA, Ricceri L, D'Anselmi F, Coluccia P, Calamandrei G, Scarpa S (2008) B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-beta deposition in mice. Mol Cell Neurosci 37:731–746
Fuso A, Nicolia V, Ricceri L, Cavallaro RA, Isopi E, Mangia F, Fiorenza MT, Scarpa S (2012) S-adenosylmethionine reduces the progress of the Alzheimer-like features induced by B-vitamin deficiency in mice. Neurobiol Aging 33(1482):e1–e16
Miller AL (2003) The methionine-homocysteine cycle and its effects on cognitive diseases. Altern Med Rev 8:7–19
Kloor D, Delabar U, Mühlbauer B, Luippold G, Osswald H (2002) Tissue levels of S-adenosylhomocysteine in the rat kidney: effects of ischemia and homocysteine. Biochem Pharmacol 63:809–815
Hermes M, von Hippel S, Osswald H, Kloor D (2005) S-adenosylhomocysteine metabolism in different cell lines: effect of hypoxia and cell density. Cell Physiol Biochem 15:233–244
Liu Q, Liu L, Zhao Y, Zhang J, Wang D, Chen J, He Y, Wu J, Zhang Z, Liu Z (2011) Hypoxia induces genomic DNA demethylation through the activation of HIF-1α and transcriptional upregulation of MAT2A in hepatoma cells. Mol Cancer Ther 10:1113–1123
Kloor D, Hermes M, Fink K, Schmid H, Klingel K, Mack A, Grenz A, Osswald H (2007) Expression and localization of S-adenosylhomocysteine-hydrolase in the rat kidney following carbon monoxide induced hypoxia. Cell Physiol Biochem 19:57–66
West RL, Lee JM, Maroun LE (1995) Hypomethylation of the amyloid precursor protein gene in the brain of an Alzheimer’s disease patient. J Mol Neurosci 6:141–146
Yang Q, Lu Z, Ramchandran R, Longo LD, Raj JU (2012) Pulmonary artery smooth muscle cell proliferation and migration in fetal lambs acclimatized to high-altitude long-term hypoxia: role of histone acetylation. Am J Physiol Lung Cell Mol Physiol 303(11):L1001–L1010. doi:10.1152/ajplung.00092.2012
Chen H, Yan Y, Davidson TL, Shinkai Y, Costa M (2006) Hypoxic stress induces dimethylated histone H3 lysine 9 through histone methyltransferase G9a in mammalian cells. Cancer Res 66:9009–9016
Costa M, Davidson TL, Chen H, Ke Q, Zhang P, Yan Y, Huang C, Kluz T (2005) Nickel carcinogenesis: epigenetics and hypoxia signalling. Mutat Res 592:79–88
Johnson AB, Denko N, Barton MC (2008) Hypoxia induces a novel signature of chromatin modifications and global repression of transcription. Mutat Res 640:174–179
Shea TB, Chan A (2008) S-adenosyl methionine: a natural therapeutic agent effective against multiple hallmarks and risk factors associated with Alzheimer’s disease. J Alzheimers Dis 13:67–70
Lee S, Lemere CA, Frost JL, Shea TB (2012) Dietary supplementation with S-adenosyl methionine delayed amyloid-β and tau pathology in 3xTg-AD mice. J Alzheimers Dis 28:423–431
Chan A, Tchantchou F, Graves V, Rozen R, Shea TB (2008) Dietary and genetic compromise in folate availability reduces acetylcholine, cognitive performance and increases aggression: critical role of S-adenosyl methionine. J Nutr Health Aging 12:252–261
Kapczinski F, Frey BN, Andreazza AC, Kauer-Sant’Anna M, Cunha AB, Post RM (2008) Increased oxidative stress as a mechanism for decreased BDNF levels in acute manic episodes. Rev Bras Psiquiatr 30:243–245
Zou J, Crews F (2006) CREB and NF-kappaB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cell Mol Neurobiol 26:385–405
Gao L, Zeng XN, Guo HM, Wu XM, Chen HJ, Di RK, Wu Y (2012) Cognitive and neurochemical alterations in hyperhomocysteinemic rat. Neurol Sci 33:39–43
Matté C, Pereira LO, Dos Santos TM, Mackedanz V, Cunha AA, Netto CA, Wyse AT (2009) Acute homocysteine administration impairs memory consolidation on inhibitory avoidance task and decreases hippocampal brain-derived neurotrophic factor immunocontent: prevention by folic acid treatment. Neurosci 163:1039–1045
Villalobos MA, De La Cruz JP, Cuerda MA, Ortiz P, Smith-Agreda JM, Sánchez De La Cuesta F (2000) Effect of S-adenosyl-l-methionine on rat brain oxidative stress damage in a combined model of permanent focal ischemia and global ischemia-reperfusion. Brain Res 883:31–40
Acknowledgments
This work was supported by National Natural Science Foundation (Nos. 81070926 and 81271310) and China 973 Preprogram (2011CB512109).
Conflict of interest statement
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, X., Sun, J., Zhang, X. et al. Epigenetic Signature of Chronic Cerebral Hypoperfusion and Beneficial Effects of S-adenosylmethionine in Rats. Mol Neurobiol 50, 839–851 (2014). https://doi.org/10.1007/s12035-014-8698-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8698-5