Abstract
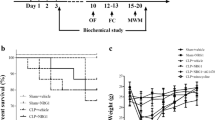
The cholinergic anti-inflammatory pathway controls the inflammatory response and nonreflexive consciousness through bidirectional communication between the brain and immune system. Moreover, brain acetylcholinesterase activity may have a role in regulating the vagus nerve in this pathway. Thus, we analyzed the role of acetylcholine (ACh) in the inflammatory response 15 days after induction of sepsis by cecal ligation and puncture (CLP). Balb/c mice were pretreated with or without donepezil (5 mg/kg/day, orally) 7 days before CLP, and mice homozygous for vesicular ACh transporter (VAChT) knockdown (KD) were subjected to CLP. All animals were sacrificed 15 days after CLP, and the plasma, spleen, and hippocampus were collected. Characterization of splenic lymphocytes and cytokine levels in the plasma, spleen, and hippocampus was determined. Our results showed a splenomegaly in group CLP. The numbers of cytotoxic T cells, helper T cells, regulatory T cells, B cells, and Th17 cells differed between mice subjected to CLP and to sham operation in both untreated and donepezil-treated groups. In VAChT-KD mice, CLP resulted in decreased cytotoxic and helper T cells and increased in Th17 cells compared with the sham. Additionally, in VAChT-KD mice, the levels of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, were increased following CLP. Thus, we concluded that ACh affected the inflammatory response at 15 days after CLP since stimulation of cholinergic transmission increased the proliferation of lymphocytes, including regulatory T cells, in association with a lower inflammatory profile and VAChT-KD decreased the number of lymphocytes and increased inflammation.



Similar content being viewed by others
References
Balk RA, Bone RC (1989) The septic syndrome. Definition and clinical implications. Crit Care Clin 5:1–8
Barbeiro DF, Barbeiro HV, Faintuchi J, Ariga SK, Mariano M, Popi AF, De Souza HP, Velasco IT, Soriano FG (2011) B-1 cells temper endotoxemic inflammatory responses. Immunobiology 216:302–308. doi:10.1016/j.imbio.2010.08.002
Lorigados CB, Soriano FG, Szabo C (2011) Pathomechanisms of myocardial dysfunction in sepsis. Endocr Metab Immune Disord Drug Targets 10:274–284. doi:10.2174/187153010791936856
Melo ES, Goloubkova T, Barbeiro DF, Gorjao R, Vasconcelos D, Szabo C, Curi R, De Lima-Salgado TM, Velasco IT, Soriano FG (2011) Endotoxin tolerance: selective alterations in gene expression and protection against lymphocyte death. Immunobiology 215:435–442. doi:10.1016/j.imbio.2009.09.002
Vandijck D, Decruyenaere JM, Blot SI (2006) The value of sepsis definitions in daily ICU-practice. Acta Clin Belg 5:220–226, http://dx.doi.org/10.1179/acb.2006.037
Hotchkiss RS, Karl IK (2003) The pathophysiology and treatment of sepsis. New Engl J Med 348:138–150. doi:10.1056/NEJMra021333
Tracey KJ (2002) The inflammatory reflex. Nature 420:853–859. doi:10.1038/nature01321
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458–462. doi:10.1038/35013070
Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, Larosa GJ, Miller EJ, Tracey KJ, Al-Abed Y (2007) Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med 35:1139–1144. doi:10.1097/01.CCM.0000259381.56526.96
Van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, De Vos AF, Tracey KJ, Van Der Poll T (2005) The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis 191:2138–2148. doi:10.1086/430323
Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L (2006) Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 203:1623–1628. doi:10.1084/jem.20052362
Gotoh M, Iguchi A, Yatomi A, Uemura K, Miura H, Futenma A, Kato K, Sakamoto N (1989) Vagally mediated insulin secretion by stimulation of brain cholinergic neurons with neostigmine in bilateral adrenalectomized rats. Brain Res 493(1):97–102. doi:10.1016/0006-8993(89)91003-2
Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, Al-Abed Y, Tracey KJ (2006) Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci U S A 103(13):5219–5223. doi:10.1073/pnas.0600506103
Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, Chavan S, Al-Abed Y, Tracey KJ (2009) Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun 23(1):41–45. doi:10.1016/j.bbi.2008.06.011
Pinheiro NM, Miranda CJ, Perini A, Câmara NO, Costa SK, Alonso-Vale MI, Caperuto LC, Tibério IF, Prado MA, Martins MA, Prado VF, Prado CM (2015) Pulmonary inflammation is regulated by the levels of the vesicular acetylcholine transporter. PLoS One 10(3):e0120441. doi:10.1371/journal.pone.0120441
Prado VF, Martins-Silva C, De Castro BM, Lima RF, Barros DM, Amaral E, Ramsey AJ, Sotnikova TD, Ramirez MR, Kim HG, Rossato JI, Koenen J, Quan H, Cota VR, Morales MF, Gomez MV, Guatimosim C, Wetsel WC, Kushmerick C, Pereira GS, Gainetdinova RR, Izquierdo I, Caron MG, Prado MA (2006) Mice deficient for the vesicular acetylcholine transporter are myasthenic and have deficits in object and social recognition. Neuron 51:601–612, http://dx.doi.org/10.1016/j.neuron.2006.08.005
Lima RF, Prado VF, Prado MA, Kushmerick C (2010) Quantal release of acetylcholine in mice with reduced levels of the vesicular acetylcholine transporter. J Neurochem 113(4):943–951. doi:10.1111/j.1471-4159.2010.06657.x
Soriano FG, Liaudet L, Szabo E, Virag L, Mabley JG, Pacher P, Szabo C (2002) Resistance to acute septic peritonitis in poly(ADP-ribose) polymerase-1-deficient mice. Shock 17:286–292
Tracey KJ (2005) Fatal sequence: the killer within. J Clin Invest 115(12):3304. doi:10.1172/JCI27259
Tracey KJ (2007) Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117(2):289–296. doi:10.1172/JCI30555
Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ (2003) The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med 9(5–8):125–134
Gallowitsch-Puerta M, Pavlov VA (2007) Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sci 80(24–25):2325–2329. doi:10.1016/j.lfs.2007.01.002
Hansen RA, Gartlehner G, Webb AP, Morgan LC, Moore CG, Jonas DE (2008) Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. Clin Interv Aging 3:211–225, http://ukpmc.ac.uk/abstract/MED/18686744
Francis PT, Palmer AM, Snape M, Wilcock GK (1999) The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry 66:137–147. doi:10.1136/jnnp.66.2.137
Giacobini E (1993) Pharmacotherapy of Alzheimer disease: new drugs and novel strategies. Prog Brain Res 98:447–454. doi:10.1016/S0079-6123(08)62431-0
Barbosa JJR, Massensini AR, Santos MS, Meireles SI, Gomez RS, Gomez MV, Romano-Silva MA, Prado VF, Prado MA (1999) Expression of the vesicular acetylcholine transporter, proteins involved in exocytosis, and functional calcium signaling in varicosities and soma of a murine septal cell line. J Neurochem 73:1881–1893. doi:10.1046/j.1471-4159.1999.01881.x
Santos MS, Barbosa J Jr, Veloso GS, Ribeiro F, Kushmerick C, Gomez MV, Ferguson SS, Prado VF, Prado MA (2001) Trafficking of green fluorescent protein tagged-vesicular acetylcholine transporter to varicosities in a cholinergic cell line. J Neurochem 78:1104–1113. doi:10.1046/j.1471-4159.2001.00494.x
Barbosa JJR, Ferreira LT, Martins-Silva C, Santos MS, Torres GE, Caron MG, Gomez MV, Ferguson SS, Prado MA, Prado VF (2002) Trafficking of the vesicular acetylcholine transporter in SN56 cells: a dynamin-sensitive step and interaction with the AP-2 adaptor complex. J Neurochem 82:1221–1228. doi:10.1046/j.1471-4159.2002.01068.x
Prado MAM, Reis RAM, Prado VF, De Mello MC, Gomez MV, De Mello FG (2002) Regulation of acetylcholine synthesis and storage. Neurochem Int 41:291–299. doi:10.1016/S0197-0186(02)00044-X
Karima R, Matsumoto S, Higashi H, Matsushima K (1999) The molecular pathogenesis of endotoxic shock and organ failure. Mol Med Today 5:123–132. doi:10.1016/S1357-4310(98)01430-0
Kunze K (2002) Metabolic encephalopathies. J Neurol 249:1150–1159. doi:10.1007/s00415-002-0869-z
Hoesel LM, Gao H, Ward PA (2006) New insights into cellular mechanisms during sepsis. Immunol Res 34:133–141. doi:10.1385/IR:34:2:133
Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ (2008) Splenic nerveis required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A 105:11008–11013. doi:10.1073/pnas.0803237105
Abbas AK (2011) Imunologia celular e molecular. Elsevier, Rio de Janeiro
Osler W (1908) Remarks on the functions of an out-patient department: made at the opening of the new out - patient department, Cardiff Infirmary, May 20th,1908. Br Med J 1:1470–1471
Pozo AL, Godrey EM, Bowles KM (2009) Splenomegaly: investigation, diagnosis and management. Blood Rev 23:105–111. doi:10.1016/j.blre.2008.10.001
Valdés-Ferrer SI, Rosas-Ballina M, Olofsson PS, Dancho ME, Ochani M, Li JH, Scheinerman JA, Katz DA, Levine YA, Hudson LK, Yang H, Pavlov VA, Roth J, Blanc L, Antoine DJ, Chavan SS, Andersson U, Diamond B, Tracey KJ (2013) HMGB1 mediates splenomegaly and expansion of splenic CD11b+ Ly-6C (high) inflammatory monocytes in murine sepsis survivors. J Intern Med 274:381–390. doi:10.1111/joim.12104
Hotchkiss RS, Monneret G, Payen D (2013) Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13:862–874. doi:10.1038/nri3552
Ayala A, Chung CS, Song GY. (1999) Lymphocyte activation, anergy, and apoptosis in polymicrobial sepsis, in: Marshall, J.C., Cohen, J. (Eds.), Immune Response in the Critically Ill. Springer, pp. 227–245. doi: 10.1007/978-3-642-57210-4_16
Mannick JÁ (1993) Trauma, sepsis, and immune defects. In: Faist E, Meakins JL, Schildberg FW (eds) Host defense dysfunction in trauma, shock and sepsis. Springer, Berlin, pp 15–21
Ayala A, Lomas JL, Grutkoski PS, Chung CS (2003) Pathological aspects of apoptosis in severe sepsis and shock? Int J Biochem Cell Biol 35:7–15. doi:10.1016/S1357-2725(02)00099-7
Gomez HG, Gonzalez SM, Londoño JM, Hoyos NA, Niño CD, Leon AL, Velilla PA, Rugeles MT, Jaimes FA (2014) Immunological characterization of compensatory anti-inflammatory response syndrome in patients with severe sepsis: a longitudinal study*. Crit Care Med 42:771–780. doi:10.1097/CCM.0000000000000100
Ono S, Kimura A, Hiraki S, Takahata R, Tsujimoto H, Kinoshita M, Miyazaki H, Yamamoto J, Hase K, Saitoh D (2013) Removal of increased circulating CD4+CD25+Foxp3+ regulatory T cells in patients with septic shock using hemoperfusion with polymyxin B-immobilized fibers. Surgery 153:262–271. doi:10.1016/j.surg.2012.06.023
Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ (2011) Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334:98–101. doi:10.1126/science.1209985
Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ (2012) Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev 248:188–204. doi:10.1111/j.1600-065X.2012.01138.x
Hofer S, Eisenbach C, Lukic IK, Schneider L, Bode K, Brueckmann M, Mautner S, Wente MN, Encke J, Werner J, Dalpke AH, Stremmel W, Nawroth PP, Martin E, Krammer PH, Bierhaus A, Weigand MA (2008) Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med 36:404–408. doi:10.1097/01.CCM.0B013E31816208B3
Acknowledgments
The authors thank FAPESP for providing financial support. We would like to thank Editage (www.editage.com.br) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeremias, I.C., Victorino, V.J., Barbeiro, H.V. et al. The Role of Acetylcholine in the Inflammatory Response in Animals Surviving Sepsis Induced by Cecal Ligation and Puncture. Mol Neurobiol 53, 6635–6643 (2016). https://doi.org/10.1007/s12035-015-9538-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9538-y