Abstract
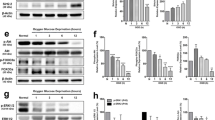
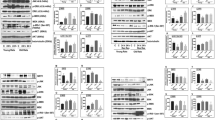
Sirtuin 2 (SIRT2) is a member of NAD+-dependent protein deacetylases involved in a wide range of pathophysiological processes including myocardial injury, Parkinson’s disease, and Huntington’s disease. However, the direct implication of SIRT2 in ischemic stroke is still unclear. In the present study, we observed that SIRT2 protein was mainly expressed in the cytoplasm of neurons, but not in astrocyte and microglia. SIRT2 was upregulated in ischemic neurons in the oxygen-glucose deprivation cell model and in the transient middle cerebral artery occlusion (tMCAo) mouse model. Moreover, expression of SIRT2 was evaluated by immunohistochemistry in human brains consisting of ischemic penumbra of cerebral stroke, and their age-matched normal controls without diagnosed neurological disorders. The results revealed that SIRT2 was mainly expressed in the cytoplasm and neurites of neurons in the brains of normal subjects, while an elevated expression and nuclear translocation of SIRT2 were detected in the ischemic penumbra of cerebral stroke. Downregulation of SIRT2 using the SIRT2-specific inhibitor AGK2 or SIRT2 knockout had neuroprotective effects in tMCAo model, which could decrease the infract volume and neurological impairment scores. In summary, our findings revealed that SIRT2 was upregulated during neuronal ischemia and translocated into neuronal nuclei, while downregulation of SIRT2 could significantly protect neurons against cerebral ischemia.





Similar content being viewed by others
References
Fisher M, Saver JL (2015) Future directions of acute ischaemic stroke therapy. Lancet Neurol 14(7):758–767. doi:10.1016/s1474-4422(15)00054-x
Lo EH, Dalkara T, Moskowitz MA (2003) Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 4(5):399–415. doi:10.1038/nrn1106
00b7d529f70dab256c000000.pdf
Moskowitz MA, Lo EH, Iadecola C (2010) The science of stroke: mechanisms in search of treatments. Neuron 67(2):181–198. doi:10.1016/j.neuron.2010.07.002
Qureshi IA, Mehler MF (2013) Understanding neurological disease mechanisms in the era of epigenetics. JAMA neurol 70(6):703–710. doi:10.1001/jamaneurol.2013.1443
Haigis MC, Sinclair DA (2010) Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 5:253–295. doi:10.1146/annurev.pathol.4.110807.092250
Frye RA (2000) Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun 273(2):793–798. doi:10.1006/bbrc.2000.3000
Donmez G, Guarente L (2010) Aging and disease: connections to sirtuins. Aging Cell 9(2):285–290. doi:10.1111/j.1474-9726.2010.00548.x
Nakagawa T, Guarente L (2011) Sirtuins at a glance. J Cell Sci 124(Pt 6):833–838. doi:10.1242/jcs.081067
Michan S, Sinclair D (2007) Sirtuins in mammals: insights into their biological function. Biochem J 404(1):1–13. doi:10.1042/BJ20070140
Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M et al (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305(5682):390–392. doi:10.1126/science.1099196
Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y (2007) Nucleocytoplasmic shuttling of the NAD + −dependent histone deacetylase SIRT1. J Biol Chem 282(9):6823–6832. doi:10.1074/jbc.M609554200
Maxwell MM, Tomkinson EM, Nobles J, Wizeman JW, Amore AM, Quinti L, Chopra V, Hersch SM et al (2011) The sirtuin 2 microtubule deacetylase is an abundant neuronal protein that accumulates in the aging CNS. Hum Mol Genet 20(20):3986–3996. doi:10.1093/hmg/ddr326
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E (2003) The human Sir2 ortholog, SIRT2, is an NAD + −dependent tubulin deacetylase. Mol Cell 11(2):437–444
Lai XJ, Ye SQ, Zheng L, Li L, Liu QR, Yu SB, Pang Y, Jin S et al (2014) Selective 14-3-3gamma induction quenches p-beta-catenin Ser37/Bax-enhanced cell death in cerebral cortical neurons during ischemia. Cell Death Dis 5:e1184. doi:10.1038/cddis.2014.152
Luthi-Carter R, Taylor DM, Pallos J, Lambert E, Amore A, Parker A, Moffitt H, Smith DL et al (2010) SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci U S A 107(17):7927–7932. doi:10.1073/pnas.1002924107
Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM et al (2007) Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317(5837):516–519. doi:10.1126/science.1143780
Li L, Liu QR, Xiong XX, Liu JM, Lai XJ, Cheng C, Pan F, Chen Y et al (2014) Neuroglobin promotes neurite outgrowth via differential binding to PTEN and Akt. Mol Neurobiol 49(1):149–162. doi:10.1007/s12035-013-8506-7
Bobrowska A, Donmez G, Weiss A, Guarente L, Bates G (2012) SIRT2 ablation has no effect on tubulin acetylation in brain, cholesterol biosynthesis or the progression of Huntington’s disease phenotypes in vivo. PLoS One 7(4):e34805. doi:10.1371/journal.pone.0034805
Liu L, Arun A, Ellis L, Peritore C, Donmez G (2014) SIRT2 enhances 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced nigrostriatal damage via apoptotic pathway. Front Aging Neurosci 6:184. doi:10.3389/fnagi.2014.00184
Pais TF, Szego EM, Marques O, Miller-Fleming L, Antas P, Guerreiro P, de Oliveira RM, Kasapoglu B et al (2013) The NAD-dependent deacetylase sirtuin 2 is a suppressor of microglial activation and brain inflammation. EMBO J 32(19):2603–2616. doi:10.1038/emboj.2013.200
Nahhas F, Dryden SC, Abrams J, Tainsky MA (2007) Mutations in SIRT2 deacetylase which regulate enzymatic activity but not its interaction with HDAC6 and tubulin. Mol Cell Biochem 303(1–2):221–230. doi:10.1007/s11010-007-9478-6
Southwood CM, Peppi M, Dryden S, Tainsky MA, Gow A (2007) Microtubule deacetylases, SirT2 and HDAC6, in the nervous system. Neurochem Res 32(2):187–195. doi:10.1007/s11064-006-9127-6
Witte H, Neukirchen D, Bradke F (2008) Microtubule stabilization specifies initial neuronal polarization. J Cell Biol 180(3):619–632. doi:10.1083/jcb.200707042
Dent EW, Gertler FB (2003) Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40(2):209–227
Fukushima N, Furuta D, Hidaka Y, Moriyama R, Tsujiuchi T (2009) Post-translational modifications of tubulin in the nervous system. J Neurochem 109(3):683–693. doi:10.1111/j.1471-4159.2009.06013.x
Pandithage R, Lilischkis R, Harting K, Wolf A, Jedamzik B, Luscher-Firzlaff J, Vervoorts J, Lasonder E et al (2008) The regulation of SIRT2 function by cyclin-dependent kinases affects cell motility. J Cell Biol 180(5):915–929. doi:10.1083/jcb.200707126
Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ (2006) Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 16(21):2166–2172. doi:10.1016/j.cub.2006.09.014
Jin YH, Kim YJ, Kim DW, Baek KH, Kang BY, Yeo CY, Lee KY (2008) Sirt2 interacts with 14-3-3 beta/gamma and down-regulates the activity of p53. Biochem Biophys Res Commun 368(3):690–695. doi:10.1016/j.bbrc.2008.01.114
Lynn EG, McLeod CJ, Gordon JP, Bao J, Sack MN (2008) SIRT2 is a negative regulator of anoxia-reoxygenation tolerance via regulation of 14-3-3 zeta and BAD in H9c2 cells. FEBS Lett 582(19):2857–2862. doi:10.1016/j.febslet.2008.07.016
Di Giovanni S, Knights CD, Rao M, Yakovlev A, Beers J, Catania J, Avantaggiati ML, Faden AI (2006) The tumor suppressor protein p53 is required for neurite outgrowth and axon regeneration. EMBO J 25(17):4084–4096. doi:10.1038/sj.emboj.7601292
Tedeschi A, Nguyen T, Puttagunta R, Gaub P, Di Giovanni S (2009) A p53-CBP/p300 transcription module is required for GAP-43 expression, axon outgrowth, and regeneration. Cell Death Differ 16(4):543–554. doi:10.1038/cdd.2008.175
Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO (2010) SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 123(Pt 24):4251–4258. doi:10.1242/jcs.073783
Calnan DR, Brunet A (2008) The FoxO code. Oncogene 27(16):2276–2288. doi:10.1038/onc.2008.21
Vaquero A, Scher MB, Lee DH, Sutton A, Cheng HL, Alt FW, Serrano L, Sternglanz R et al (2006) SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev 20(10):1256–1261. doi:10.1101/gad.1412706
Li W, Zhang B, Tang J, Cao Q, Wu Y, Wu C, Guo J, Ling EA et al (2007) Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J Neurosci Off J Soc Neurosci 27(10):2606–2616. doi:10.1523/JNEUROSCI.4181-06.2007
Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J et al (2010) Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol 12(7):665–675. doi:10.1038/ncb2069
Wang F, Nguyen M, Qin FX, Tong Q (2007) SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 6(4):505–514. doi:10.1111/j.1474-9726.2007.00304.x
Wang T, Gu J, Wu PF, Wang F, Xiong Z, Yang YJ, Wu WN, Dong LD et al (2009) Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-kappaB pathways and inhibition of intracellular ROS/RNS generation. Free Radic Biol Med 47(3):229–240. doi:10.1016/j.freeradbiomed.2009.02.027
Donmez G, Outeiro TF (2013) SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 5(3):344–352. doi:10.1002/emmm.201302451
Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H et al (2010) Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 122(21):2170–2182. doi:10.1161/CIRCULATIONAHA.110.958033
Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, Akaike A (2007) Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol 73(4):550–560. doi:10.1016/j.bcp.2006.11.003
Pallos J, Bodai L, Lukacsovich T, Purcell JM, Steffan JS, Thompson LM, Marsh JL (2008) Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a drosophila model of Huntington’s disease. Hum Mol Genet 17(23):3767–3775. doi:10.1093/hmg/ddn273
Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Neri C (2005) Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet 37(4):349–350. doi:10.1038/ng1534
Anekonda TS (2006) Resveratrol—a boon for treating Alzheimer’s disease? Brain Res Rev 52(2):316–326. doi:10.1016/j.brainresrev.2006.04.004
Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE (2009) Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int 54(2):111–118. doi:10.1016/j.neuint.2008.10.008
Acknowledgments
This work was supported by grants from the National Nature Science Foundation of China (Nos. 81172397, 81471386, and 81672504 to X.Q. Chen and Nos. 31571044 and 31371384 to B. Tian). We thank Lei Pei (Tongji Medical School, Huazhong University of Science and Technology) for the technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal handling and experiments were performed in accordance with NIH guidelines and reviewed by the Ethics Committees of Huazhong University of Science and Technology (HUST).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Xiao Qiang Xie and Pei Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xie, X.Q., Zhang, P., Tian, B. et al. Downregulation of NAD-Dependent Deacetylase SIRT2 Protects Mouse Brain Against Ischemic Stroke. Mol Neurobiol 54, 7251–7261 (2017). https://doi.org/10.1007/s12035-016-0173-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0173-z