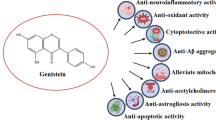
Abstract
Alzheimer’s disease (AD) is a devastating brain disorder characterized by an increased level of amyloid-beta (Aβ) peptide deposition and neuronal cell death leading to an impairment of learning and thinking skills. The Aβ deposition is a key factor in senile plaques of the AD brain which cause the elevation of intracellular calcium ions and the production of formidable free radicals, both of which greatly contribute to the AD-associated cascade, leading to unstoppable neuronal loss in the hippocampal region of the brain. Natural products are currently considered as an alternative strategy for the discovery of novel multipotent drugs against AD. They include the naturally occurring dietary soy isoflavone genistein which has been recognized to possess several health-promoting effects. Genistein has been mainly focused because of its potential on amelioration of Aβ-induced impairment and its antioxidant capacity to scavenge the free radicals produced in AD. It can also directly interact with the targeted signaling proteins and stabilize their activity to prevent AD. An improved understanding of the direct interactions between genistein and target proteins would contribute to the further development of AD treatment. This review mainly focuses on molecular targets and the therapeutic effects regulated by genistein, which has the ability to directly target the Aβ peptide and to control its activity involved in intracellular signaling pathways, which otherwise would lead to neuronal death in the hippocampal region of the AD brain.


Similar content being viewed by others
References
Yoo KY, Park SY (2012) Terpenoids as potential anti-Alzheimer’s disease therapeutics. Molecules 17(3):3524–3538
Geldmacher DS (2007) Treatment guidelines for Alzheimer’s disease: redefining perceptions in primary care. Prim Care Companion J Clin Psychiatry 9(2):113–121
Schmitt FA, Wichems CH (2006) A systematic review of assessment and treatment of moderate to severe Alzheimer’s disease. Prim Care Companion J Clin Psychiatry 8(3):158–159
Terry AV, Buccafusco JJ (2003) The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther 306(3):821–827
Mecocci P, Bladström A, Stender K (2009) Effects of memantine on cognition in patients with moderate to severe Alzheimer’s disease: post-hoc analyses of ADAS-cog and SIB total and single-item scores from six randomized, double-blind, placebo-controlled studies. Int J Geriatr Psychiatry 24(5):532–538
Deardorff WJ, Feen E, Grossberg GT (2015) The use of cholinesterase inhibitors across all stages of Alzheimer’s disease. Drugs Aging 32(7):537–547
Doody R, Stevens J, Beck C, Dubinsky R, Kaye J, Gwyther L, Mohs RC, Thal LJ et al (2001) Practice parameter: management of dementia (an evidence-based review) report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 56(9):1154–1166
Rafii MS, Aisen PS (2009) Recent developments in Alzheimer’s disease therapeutics. BMC Med 7:7
Tsao R (2010) Chemistry and biochemistry of dietary polyphenols. Nutrients 2(12):1231–1246
Martorell M, Forman K, Castro N, Capó X, Tejada S, Sureda A (2016) Potential therapeutic effects of oleuropein aglycone in Alzheimer’s disease. Curr Pharm Biotechnol 17(11):994–1001
Zhang H, Tsao R (2016) Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci 8:33–42
Lampe JW (2003) Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J Nutr 133(3):956S–964S
Abdallah HH, Mavri J, Repič M, Lee VS, Wahab HA (2012) Chemical reaction of soybean flavonoids with DNA: a computational study using the implicit solvent model. Int J Mol Sci 13(2):1269–1283
Glazier MG, Bowman MA (2001) A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch Intern Med 161(9):1161–1172
Xi YD, Li XY, Ding J, Yu HL, Ma WW, Yuan LH, Wu J, Xiao R (2013) Soy isoflavone alleviates Aβ1-42-induced impairment of learning and memory ability through the regulation of RAGE/LRP-1 in neuronal and vascular tissue. Curr Neurovasc Res 10(2):144–156
Xi YD, Li XY, Yu HL, Jing H, Ma WW, Yuan LH, Zhang DD, Wu J et al (2014) Soy isoflavone antagonizes the oxidative cerebrovascular injury induced by β-amyloid peptides 1–42 in rats. Neurochem Res 39(7):1374–1381
Xu ML, Liu J, Zhu C, Gao Y, Zhao S, Liu W, Zhang Y (2015) Interactions between soy isoflavones and other bioactive compounds: a review of their potentially beneficial health effects. Phytochem Rev 14(3):459–467
Dixon RA, Ferreira D (2002) Genistein. Phytochemistry 60(3):205–211
Zubik L, Meydani M (2003) Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr 77(6):1459–1465
Xu X, Wang HJ, Murphy PA, Hendrich S (2000) Neither background diet nor type of soy food affects short-term isoflavone bioavailability in women. J Nutr 130(4):798–801
Liggins J, Bluck LJ, Runswick S, Atkinson C, Coward WA, Bingham SA (2000) Daidzein and genistein content of fruits and nuts. J Nutr Biochem 11(6):326–331
Murphy PA, Wang HJ (1993) Antiproliferative effect of genistein and adriamycin against estrogen-dependent and -independent human breast carcinoma cell lines. Total genistein, daidzein and glycitein content of soyfoods presented to the 18th National Nutrient Databank Conference Baton Rouge, LA
BCERC COTC fact sheet—early life exposure to the phytoestrogen genistein and breast cancer risk in later years; Phytoestrogen Daidzein, 11/07/2007
Bhagwat S, Haytowitz DB, Holden JM (2008) USDA database for the isoflavone content of selected foods release 2.0. US Department of Agriculture, Maryland, p. 15
Liu R, Hu Y, Li J, Lin Z (2007) Production of soybean isoflavone genistein in non-legume plants via genetically modified secondary metabolism pathway. Metab Eng 9(1):1–7
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79(5):727–747
Park YK, Aguiar CL, Alencar SM, Mascarenhas HAA, Scamparini ARP (2002) Conversion of malonyl-beta-glycoside isoflavones into glycoside isoflavones in brazilian soybeans. Food Sci Technol 22(2):130–135
Supko JG, Malspeis L (1995) Plasma pharmacokinetics of genistein in mice. Int J Oncol 7(4):847–854
Yang Z, Kulkarni K, Zhu W, Hu M (2012) Bioavailability and pharmacokinetics of genistein: mechanistic studies on its ADME. Anti Cancer Agents Med Chem 12(10):1264–1280
Piskula MK, Yamakoshi J, Iwai Y (1999) Daidzein and genistein but not their glucosides are absorbed from the rat stomach. FEBS Lett 447(2):287–291
Steensma A, Faassen-Peters MA, Noteborn HP, Rietjens IM (2006) Bioavailability of genistein and its glycoside genistin as measured in the portal vein of freely moving unanesthetized rats. J Agric Food Chem 54(21):8006–8012
Setchell KD, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, Heubi JE (2002) Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutri 76(2):447–453
Fischer L, Mahoney C, Jeffcoat AR, Koch MA, Thomas BF, Valentine JL, Stinchcombe T, Boan J et al (2004) Clinical characteristics and pharmacokinetics of purified soy isoflavones: multiple-dose administration to men with prostate neoplasia. Nutr Cancer 48(2):160–170
Clarke DB, Lloyd AS, Botting NP, Oldfield MF, Needs PW, Wiseman H (2002) Measurement of intact sulfate and glucuronide phytoestrogen conjugates in human urine using isotope dilution liquid chromatography-tandem mass spectrometry with [13C3] isoflavone internal standards. Anal Biochem 309(1):158–172
Manach C, Williamson G, Morand C, Scalbert A, Rémésy C (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81(1):230S–242S
Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A et al (2001) Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr 131(4):1362S–1375S
Rowland I, Faughnan M, Hoey L, Wähälä K, Williamson G, Cassidy A (2003) Bioavailability of phyto-oestrogens. Br J Nutr 89(S1):S45–S58
Messina M, McCaskill-Stevens W, Lampe JW (2006) Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst 98(18):1275–1284
Center of Disease Control and prevention (2005) Third national report on human exposure to environmental chemicals: executive summary. Third national report on human exposure to environmental chemicals: executive summary. NCEH; 2005
Nabavi SF, Daglia M, Tundis R, Rosa Loizzo M, Sobarzo-Sanchez E, Erdogan Orhan I, Nabavi SM (2015) Genistein: a boon for mitigating ischemic stroke. Curr topics med chem 15(17):1714–1721
Ganai AA, Farooqi H (2015) Bioactivity of genistein: a review of in vitro and in vivo studies. Biomed Pharmacother 76:30–38
Kim IG, Kim JS, Lee JH, Cho EW (2014) Genistein decreases cellular redox potential, partially suppresses cell growth in HL-60 leukemia cells and sensitizes cells to γ-radiation-induced cell death. Mol Med Rep 10(6):2786–2792
Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S-i, Itoh N, Shibuya M, Fukami Y (1987) Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262(12):5592–5595
Wei H, Bowen R, Cai Q, Barnes S, Wang Y (1995) Antioxidant and antipromotional effects of the soybean isoflavone genistein. Exp Biol Med 208(1):124–130
Park YJ, Jang YM, Kwon YH (2009) Isoflavones prevent endoplasmic reticulum stress-mediated neuronal degeneration by inhibiting tau hyperphosphorylation in SH-SY5Y cells. J Med Food 12(3):528–535
Fotsis T, Pepper M, Adlercreutz H, Fleischmann G, Hase T, Montesano R, Schweigerer L (1993) Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci 90(7):2690–2694
Soni M, White L, Kridawati A, Bandelow S, Hogervorst E (2016) Phytoestrogen consumption and risk for cognitive decline and dementia: with consideration of thyroid status and other possible mediators. J Steroid Biochem Mol Biol 160:67–77
Lephart ED, West TW, Weber KS, Rhees RW, Setchell KD, Adlercreutz H, Lund TD (2002) Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol 24(1):5–16
Bansal N, Parle M (2010) Soybean supplementation helps reverse age-and scopolamine-induced memory deficits in mice. J Med Food 13(6):1293–1300
Safahani M, Amani R, Aligholi H, Sarkaki A, Badavi M, Zand Moghaddam A, Haghighizadeh MH (2011) Effect of different doses of soy isoflavones on spatial learning and memory in ovariectomized rats. Basic Clin Neurosci 2(4):12–18
Omoni AO, Aluko RE (2005) Soybean foods and their benefits: potential mechanisms of action. Nutr Rev 63(8):272–283
Spagnuolo C, Russo GL, Orhan IE, Habtemariam S, Daglia M, Sureda A, Nabavi SF, Devi KP et al (2015) Genistein and cancer: current status, challenges, and future directions. Adv Nutr 6(4):408–419
Wang TT, Sathyamoorthy N, Phang JM (1996) Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis 17(2):271–275
Mazumder MAR, Hongsprabhas P (2016) Genistein as antioxidant and antibrowning agents in in vivo and in vitro: a review. Biomedicine Pharmacother 82:379–392
Wiegand H, Wagner AE, Boesch-Saadatmandi C, Kruse HP, Kulling S, Rimbach G (2009) Effect of dietary genistein on phase II and antioxidant enzymes in rat liver. Cancer Genomics-Proteomics 6(2):85–92
Suzuki K, Koike H, Matsui H, Ono Y, Hasumi M, Nakazato H, Okugi H, Sekine Y et al (2002) Genistein, a soy isoflavone, induces glutathione peroxidase in the human prostate cancer cell lines LNCaP and PC-3. Int J Cancer 99(6):846–852
López T, López S, Arias CF (2015) The tyrosine kinase inhibitor genistein induces the detachment of rotavirus particles from the cell surface. Virus Res 210:141–148
Banerjee S, Li Y, Wang Z, Sarkar FH (2008) Multi-targeted therapy of cancer by genistein. Cancer Lett 269(2):226–242
Ogawara H, Akiyama T, Watanabe SI, Ito N, Kobori M, Seoda Y (1989) Inhibition of tyrosine protein kinase activity by synthetic isoflavones and flavones. J Antibiotics 42(2):340–343
Pan Y, Anthony M, Clarkson TB (1999) Effect of estradiol and soy phytoestrogens on choline acetyltransferase and nerve growth factor mRNAs in the frontal cortex and hippocampus of female rats. Exp Biol Med 221(2):118–125
File SE, Hartley DE, Alom N, Rattray M (2003) Soya phytoestrogens change cortical and hippocampal expression of BDNF mRNA in male rats. Neurosci Lett 338(2):135–138
File SE, Jarrett N, Fluck E, Duffy R, Casey K, Wiseman H (2001) Eating soya improves human memory. Psychopharmacology 157(4):430–436
Bagheri M, Joghataei MT, Mohseni S, Roghani M (2011) Genistein ameliorates learning and memory deficits in amyloid β (1–40) rat model of Alzheimer’s disease. Neurobiol Learn Mem 95(3):270–276
Chan WH, Yu JS (2000) Inhibition of UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermal carcinoma A 431 cells by genistein. J Cell Biochem 78(1):73–84
Kurzer MS, Xu X (1997) Dietary phytoestrogens. Ann Rev Nutr 17(1):353–381
Andersen JM, Myhre O, Fonnum F (2003) Discussion of the role of the extracellular signal-regulated kinase-phospholipase A2 pathway in production of reactive oxygen species in Alzheimer’s disease. Neurochem Res 28(2):319–326
Zeng H, Chen Q, Zhao B (2004) Genistein ameliorates β-amyloid peptide (25–35)-induced hippocampal neuronal apoptosis. Free Radical Bio Med 36(2):180–188
Zhao L, Woody SK, Chhibber A (2015) Estrogen receptor β in Alzheimer’s disease: from mechanisms to therapeutics. Ageing Res Rev 24:178–190
Bang OY, Hong HS, Kim DH, Kim H, Boo JH, Huh K, Mook-Jung I (2004) Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity. Neurobiol DisN 16(1):21–28
Morán J, Garrido P, Alonso A, Cabello E, González C (2013) 17β-estradiol and genistein acute treatments improve some cerebral cortex homeostasis aspects deteriorated by aging in female rats. Exp Gerontol 48(4):414–421
Donzelli A, Braida D, Finardi A, Capurro V, Valsecchi AE, Colleoni M, Sala M (2010) Neuroprotective effects of genistein in mongolian gerbils: estrogen receptor-. BETA. Involvement. J Pharmacol Sci 114(2):158–167
Selkoe DJ (1996) Amyloid β-protein and the genetics of Alzheimer’s disease. J Biol Chem 271(31):18295–18298
Butterfield DA, Lauderback CM (2002) Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress 1, 2. Free Radic Biol Med 32(11):1050–1060
Roberson ED, Mucke L (2006) 100 years and counting: prospects for defeating Alzheimer’s disease. Science 314(5800):781–784
Kim C, Ye F, Ginsberg MH (2011) Regulation of integrin activation. Ann Rev Cell Dev Biol 27:321–345
Liao W, Jin G, Zhao M, Yang H (2013) The effect of genistein on the content and activity of α-and β-secretase and protein kinase c in aβ-injured hippocampal neurons. Basic clin pharmacol toxicol 112(3):182–185
Okumura N, Yoshida H, Nishimura Y, Murakami M, Kitagishi Y, Matsuda S (2012) Genistein downregulates presenilin 1 and ubiquilin 1 expression. Mol Med Rep 5(2):559–561
Radi E, Formichi P, Battisti C, Federico A (2014) Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimers Dis 42:S125–S152
Ma W, Yuan L, Yu H, Ding B, Xi Y, Feng J, Xiao R (2010) Genistein as a neuroprotective antioxidant attenuates redox imbalance induced by β-amyloid peptides 25–35 in PC12 cells. Int J Dev Neurosci 28(4):289–295
Linford NJ, Dorsa DM (2002) 17β-estradiol and the phytoestrogen genistein attenuate neuronal apoptosis induced by the endoplasmic reticulum calcium-ATPase inhibitor thapsigargin. Steroids 67(13):1029–1040
Yu HL, Li L, Zhang XH, Xiang L, Zhang J, Feng JF, Xiao R (2009) Neuroprotective effects of genistein and folic acid on apoptosis of rat cultured cortical neurons induced by β-amyloid 31-35. Br J Nutr 102(05):655–662
Ding B, Yuan L, Yu H, Li L, Ma W, Bi Y, Feng J, Xiao R (2011) Genistein and folic acid prevent oxidative injury induced by β-amyloid peptide. Basic Clin Pharmacol Toxicol 108(5):333–340
Munoz L, Ammit AJ (2010) Targeting p38 MAPK pathway for the treatment of Alzheimer’s disease. Neuropharmacology 58(3):561–568
Valles SL, Dolz-Gaiton P, Gambini J, Borras C, LLoret A, Pallardo FV, Viña J (2010) Estradiol or genistein prevent Alzheimer’s disease-associated inflammation correlating with an increase PPARγ expression in cultured astrocytes. Brain Res 1312:138–144
Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G (2010) Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim Biophysic Acta (BBA)-Molecular Basis of Disease 1802(1):2–10
Xi YD, Yu HL, Ma WW, Ding BJ, Ding J, Yuan LH, Feng JF, Xiao R (2011) Genistein inhibits mitochondrial-targeted oxidative damage induced by beta-amyloid peptide 25–35 in PC12 cells. J Bioenerg Biomembr 2011 43(4):399–407
Ma WW, Hou CC, Zhou X, Yu HL, Ding J, Zhao X, Xiao R (2013) Genistein alleviates the mitochondria-targeted DNA damage induced by β-amyloid peptides 25–35 in C6 glioma cells. Neurochem Res 38(7):1315–1323
White FA, Bhangoo SK, Miller RJ (2005) Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov 4(10):834–844
Heneka MT, Reyes-Irisarri E, Hull M, Kummer MP (2011) Impact and therapeutic potential of PPARs in Alzheimer’s disease. Curr Neuropharmacol 9(4):643–650
Facci L, Barbierato M, Marinelli C, Argentini C, Skaper SD, Giusti P (2014) Toll-like receptors 2,-3 and-4 prime microglia but not astrocytes across central nervous system regions for ATP-dependent interleukin-1 [bgr] release. Sci Rep 4:6824
Yu HL, Li XY, Zhou X, Yuan LH, Ma WW, Xi YD, Zhao X, Wu J et al (2013) Beta amyloid peptide (25-35) leading to inflammation through toll-like receptors and the anti-inflammatory effect of genistein in BV-2 cells. J Mol Neurosci 51(3):771–778
Zhou X, Yuan L, Zhao X, Hou C, Ma W, Yu H, Xiao R (2014) Genistein antagonizes inflammatory damage induced by β-amyloid peptide in microglia through TLR4 and NF-κB. Nutrition 30(1):90–95
Ma W, Ding B, Yu H, Yuan L, Xi Y, Xiao R (2015) Genistein alleviates β-amyloid-induced inflammatory damage through regulating toll-like receptor 4/nuclear factor κB. J Med Food 18(3):273–279
Crystal AS, Shaw AT, Sequist LV, Friboulet L, Niederst MJ, Lockerman EL, Frias RL, Gainor JF et al (2014) Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science 346(6216):1480–1486
Iadecola C, Gorelick PB (2003) Converging pathogenic mechanisms in vascular and neurodegenerative dementia. Stroke 34(2):335–337
Xi YD, Yu HL, Ding J, Ma WW, Yuan LH, Feng JF, Xiao YX, Xiao R (2012) Flavonoids protect cerebrovascular endothelial cells through Nrf2 and PI3K from β-amyloid peptide-induced oxidative damage. Curr Neurovasc Res 9(1):32–41
Steinmetz KL, Spack EG (2009) The basics of preclinical drug development for neurodegenerative disease indications. BMC Neurol 9(1):1
Pan Y, Anthony M, Watson S, Clarkson TB (2000) Soy phytoestrogens improve radial arm maze performance in ovariectomized retired breeder rats and do not attenuate benefits of 17 [beta]-estradiol treatment. Menopause 7(4):230–235
Sarkaki A, Amani R, Badavi M, Moghaddam AZ, Aligholi H, Safahani, Haghighizadeh MH (2008) Pre-treatment effect of different doses of soy isoflavones on spatial learning and memory in an ovariectomized animal model of Alzheimer’s disease. Pak J Biol Sci 11(8):1114–1119
Bagheri M, Roghani M, Joghataei MT, Mohseni S (2012) Genistein inhibits aggregation of exogenous amyloid-beta 1–40 and alleviates astrogliosis in the hippocampus of rats. Brain Res 1429:145–154
Bagheri M, Rezakhani A, Nyström S, Turkina MV, Roghani M, Hammarström P, Mohseni S (2013) Amyloid beta 1-40-induced astrogliosis and the effect of genistein treatment in rat: a three-dimensional confocal morphometric and proteomic study. PLoS One 8(10):e76526
Li R, He P, Cui J, Staufenbiel M, Harada N, Shen Y (2013) Brain endogenous estrogen levels determine responses to estrogen replacement therapy via regulation of BACE1 and NEP in female Alzheimer’s transgenic mice. Mol Neurobiol 47(3):857–867
Bonet-Costa V, Herranz-Pérez V, Blanco-Gandía M, Mas-Bargues C, Inglés M, Garcia-Tarraga P, Rodriguez-Arias M, Miñarro J et al Clearing amyloid-β through PPARγ/ApoE activation by genistein is a treatment of experimental Alzheimer’s disease. J Alzheimers Dis 51(3):701–711
Gutierrez-Zepeda A, Santell R, Wu Z, Brown M, Wu Y, Khan I, Link CD, Zhao B et al (2005) Soy isoflavone glycitein protects against beta amyloid-induced toxicity and oxidative stress in transgenic Caenorhabditis elegans. BMC Neurosci 6(1):54
Kohara Y, Kawaguchi S, Kuwahara R, Uchida Y, Oku Y, Yamashita K (2015) Genistein improves spatial learning and memory in male rats with elevated glucose level during memory consolidation. Physiol Behav 140:15–22
Barrett-Connor E, Stuenkel CA (2001) Hormone replacement therapy (HRT)—risks and benefits. Int J Epidemiol 30(3):423–426
Pan M, Han H, Zhong C, Geng Q (2012) Effects of genistein and daidzein on hippocampus neuronal cell proliferation and BDNF expression in H19-7 neural cell line. J Nutr Health Aging 16(4):389–394
Lee JH, Jiang Y, Han DH, Shin SK, Choi WH, Lee MJ (2014) Targeting estrogen receptors for the treatment of Alzheimer’s disease. Mol Neurobiol 49(1):39–49
McClain RM, Wolz E, Davidovich A, Pfannkuch F, Edwards JA, Bausch J (2006) Acute, subchronic and chronic safety studies with genistein in rats. Food Chem Toxicol 44(1):56–80
Acknowledgments
The Indian authors gladly acknowledge the computational and bioinformatics facility provided by the Alagappa University Bioinformatics Infrastructure Facility (funded by Department of Biotechnology, Government of India; Grant No. BT/BI/25/015/2012).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Devi, K.P., Shanmuganathan, B., Manayi, A. et al. Molecular and Therapeutic Targets of Genistein in Alzheimer’s Disease. Mol Neurobiol 54, 7028–7041 (2017). https://doi.org/10.1007/s12035-016-0215-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0215-6