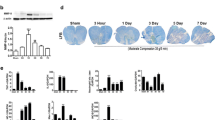
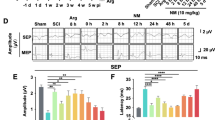
Abstract
The blood-spinal cord barrier (BSCB) is a specialized protective barrier that regulates the movement of molecules between blood vessels and the spinal cord parenchyma. Analogous to the blood-brain barrier (BBB), the BSCB plays a crucial role in maintaining the homeostasis and internal environmental stability of the central nervous system (CNS). After spinal cord injury (SCI), BSCB disruption leads to inflammatory cell invasion such as neutrophils and macrophages, contributing to permanent neurological disability. In this review, we focus on the major proteins mediating the BSCB disruption or BSCB repair after SCI. This review is composed of three parts. Section 1. SCI and the BSCB of the review describes critical events involved in the pathophysiology of SCI and their correlation with BSCB integrity/disruption. Section 2. Major proteins involved in BSCB disruption in SCI focuses on the actions of matrix metalloproteinases (MMPs), tumor necrosis factor alpha (TNF-α), heme oxygenase-1 (HO-1), angiopoietins (Angs), bradykinin, nitric oxide (NO), and endothelins (ETs) in BSCB disruption and repair. Section 3. Therapeutic approaches discusses the major therapeutic compounds utilized to date for the prevention of BSCB disruption in animal model of SCI through modulation of several proteins.


Similar content being viewed by others
References
Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M (2011) The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol 70(2):194–206. doi:10.1002/ana.22421
Bazzoni G, Dejana E (2004) Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 84(3):869–901. doi:10.1152/physrev.00035.2003
Begley DJ, Brightman MW (2003) Structural and functional aspects of the blood-brain barrier. Prog Drug Res 61:39–78
Lee JY, Kim HS, Choi HY, Oh TH, Yune TY (2012) Fluoxetine inhibits matrix metalloprotease activation and prevents disruption of blood-spinal cord barrier after spinal cord injury. Brain J Neurol 135(Pt 8):2375–2389. doi:10.1093/brain/aws171
Palmer AM (2013) Multiple sclerosis and the blood-central nervous system barrier. Cardiovasc Psychiatry Neurol 2013:530356. doi:10.1155/2013/530356
Vincent T, Saikali P, Cayrol R, Roth AD, Bar-Or A, Prat A, Antel JP (2008) Functional consequences of neuromyelitis optica-IgG astrocyte interactions on blood-brain barrier permeability and granulocyte recruitment. J Immunol 181(8):5730–5737
Garbuzova-Davis S, Saporta S, Sanberg PR (2008) Implications of blood-brain barrier disruption in ALS. Amyotroph Lateral Scler 9(6):375–376
Hemley SJ, Tu J, Stoodley MA (2009) Role of the blood-spinal cord barrier in posttraumatic syringomyelia. J Neurosurg Spine 11(6):696–704. doi:10.3171/2009.6.SPINE08564
Cahill LS, Laliberté CL, Liu XJ, Bishop J, Nieman BJ, Mogil JS, Sorge RE, Jones CD et al (2014) Quantifying blood-spinal cord barrier permeability after peripheral nerve injury in the living mouse. Mol Pain 10(1):60
Li XQ, Lv HW, Wang ZL, Tan WF, Fang B, Ma H (2015) MiR-27a ameliorates inflammatory damage to the blood-spinal cord barrier after spinal cord ischemia: reperfusion injury in rats by downregulating TICAM-2 of the TLR4 signaling pathway. J Neuroinflammation 12(1):25. doi:10.1186/s12974-015-0246-3
Maikos JT, Shreiber DI (2007) Immediate damage to the blood-spinal cord barrier due to mechanical trauma. J Neurotrauma 24(3):492–507. doi:10.1089/neu.2006.0149
Noble LJ, Wrathall JR (1989) Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res 482(1):57–66
Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ (2003) Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res 74(2):227–239. doi:10.1002/jnr.10759
Rossignol S, Schwab M, Schwartz M, Fehlings MG (2007) Spinal cord injury: time to move? J Neurosci Off J Soc Neurosci 27(44):11782–11792. doi:10.1523/JNEUROSCI.3444-07.2007
Acarin L, Gonzalez B, Castellano B (2000) Neuronal, astroglial and microglial cytokine expression after an excitotoxic lesion in the immature rat brain. Eur J Neurosci 12(10):3505–3520
Bartholdi D, Schwab ME (1997) Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci 9(7):1422–1438
Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E (2000) Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J Neurotrauma 17(3):203–218
Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS (2001) Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 24(5):254–264
Tator CH (1996) Experimental and clinical studies of the pathophysiology and management of acute spinal cord injury. J Spinal Cord Med 19(4):206–214
Grossman SD, Rosenberg LJ, Wrathall JR (2001) Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp Neurol 168(2):273–282. doi:10.1006/exnr.2001.7628
Beattie MS, Hermann GE, Rogers RC, Bresnahan JC (2002) Cell death in models of spinal cord injury. Prog Brain Res 137:37–47
Degeneration and regeneration of the nervous system (1928) Oxford UP
Clemente CD, Windle WF (1954) Regeneration of severed nerve fibers in the spinal cord of the adult cat. J Comp Neurol 101(3):691–731
Liuzzi FJ, Lasek RJ (1987) Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science 237(4815):642–645
Rudge JS, Silver J (1990) Inhibition of neurite outgrowth on astroglial scars in vitro. J Neurosci Off J Soc Neurosci 10(11):3594–3603
Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57(2):173–185. doi:10.1124/pr.57.2.4
Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57(2):178–201. doi:10.1016/j.neuron.2008.01.003
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7(1):41–53. doi:10.1038/nrn1824
Popovich PG, Horner PJ, Mullin BB, Stokes BT (1996) A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp Neurol 142(2):258–275. doi:10.1006/exnr.1996.0196
Cohen DM, Patel CB, Ahobila-Vajjula P, Sundberg LM, Chacko T, Liu SJ, Narayana PA (2009) Blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced MRI. NMR Biomed 22(3):332–341. doi:10.1002/nbm.1343
Runge VM, Wells JW, Baldwin SA, Scheff SW, Blades DA (1997) Evaluation of the temporal evolution of acute spinal cord injury. Investig Radiol 32(2):105–110
Figley SA, Khosravi R, Legasto JM, Tseng YF, Fehlings MG (2014) Characterization of vascular disruption and blood-spinal cord barrier permeability following traumatic spinal cord injury. J Neurotrauma 31(6):541–552. doi:10.1089/neu.2013.3034
Ankeny DP, Popovich PG (2009) Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience 158(3):1112–1121. doi:10.1016/j.neuroscience.2008.07.001
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5(2):146–156. doi:10.1038/nrn1326
Leal-Filho MB (2011) Spinal cord injury: from inflammation to glial scar. Surg Neurol Int 2:112. doi:10.4103/2152-7806.83732
Aube B, Levesque SA, Pare A, Chamma E, Kebir H, Gorina R, Lecuyer MA, Alvarez JI et al (2014) Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J Immunol 193(5):2438–2454. doi:10.4049/jimmunol.1400401
Noble LJ, Wrathall JR (1988) Blood-spinal cord barrier disruption proximal to a spinal cord transection in the rat: time course and pathways associated with protein leakage. Exp Neurol 99(3):567–578
Tator CH, Fehlings MG (1991) Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 75(1):15–26. doi:10.3171/jns.1991.75.1.0015
Noble LJ, Mautes AE, Hall JJ (1996) Characterization of the microvascular glycocalyx in normal and injured spinal cord in the rat. J Comp Neurol 376(4):542–556. doi:10.1002/(SICI)1096-9861(19961223)376:4<542::AID-CNE4>3.0.CO;2-1
Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z (2002) Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci Off J Soc Neurosci 22(17):7526–7535
Stirling DP, Yong VW (2008) Dynamics of the inflammatory response after murine spinal cord injury revealed by flow cytometry. J Neurosci Res 86(9):1944–1958. doi:10.1002/jnr.21659
Simard JM, Woo SK, Norenberg MD, Tosun C, Chen Z, Ivanova S, Tsymbalyuk O, Bryan J et al (2010) Brief suppression of Abcc8 prevents autodestruction of spinal cord after trauma. Sci Transl Med 2(28):28ra29. doi:10.1126/scitranslmed.3000522
Winkler EA, Sengillo JD, Sagare AP, Zhao Z, Ma Q, Zuniga E, Wang Y, Zhong Z et al (2014) Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc Natl Acad Sci U S A 111(11):E1035–E1042. doi:10.1073/pnas.1401595111
Sharma HS (2011) Early microvascular reactions and blood-spinal cord barrier disruption are instrumental in pathophysiology of spinal cord injury and repair: novel therapeutic strategies including nanowired drug delivery to enhance neuroprotection. J Neural Transm 118(1):155–176. doi:10.1007/s00702-010-0514-4
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463
Werb Z (1997) ECM and cell surface proteolysis: regulating cellular ecology. Cell 91(4):439–442
Rosenberg GA, Estrada EY, Dencoff JE (1998) Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke 29(10):2189–2195
Liu W, Hendren J, Qin XJ, Shen J, Liu KJ (2009) Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia. J Neurochem 108(3):811–820. doi:10.1111/j.1471-4159.2008.05821.x
Gurney KJ, Estrada EY, Rosenberg GA (2006) Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis 23(1):87–96. doi:10.1016/j.nbd.2006.02.006
Mautes AE, Weinzierl MR, Donovan F, Noble LJ (2000) Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther 80(7):673–687
Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L (1998) Acute inflammatory response in spinal cord following impact injury. Exp Neurol 151(1):77–88. doi:10.1006/exnr.1998.6785
Caron A, Desrosiers RR, Beliveau R (2005) Ischemia injury alters endothelial cell properties of kidney cortex: stimulation of MMP-9. Exp Cell Res 310(1):105–116. doi:10.1016/j.yexcr.2005.07.004
Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA (2007) Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27(4):697–709. doi:10.1038/sj.jcbfm.9600375
Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, Noble-Haeusslein LJ (2006) Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci Off J Soc Neurosci 26(39):9841–9850. doi:10.1523/JNEUROSCI.1993-06.2006
Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S, Yong VW (2003) An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J Neurosci Off J Soc Neurosci 23(31):10107–10115
Zuo J, Ferguson TA, Hernandez YJ, Stetler-Stevenson WG, Muir D (1998) Neuronal matrix metalloproteinase-2 degrades and inactivates a neurite-inhibiting chondroitin sulfate proteoglycan. J Neurosci Off J Soc Neurosci 18(14):5203–5211
Lee JY, Choi HY, Ahn HJ, Ju BG, Yune TY (2014) Matrix metalloproteinase-3 promotes early blood-spinal cord barrier disruption and hemorrhage and impairs long-term neurological recovery after spinal cord injury. Am J Pathol 184(11):2985–3000. doi:10.1016/j.ajpath.2014.07.016
Yu F, Kamada H, Niizuma K, Endo H, Chan PH (2008) Induction of mmp-9 expression and endothelial injury by oxidative stress after spinal cord injury. J Neurotrauma 25(3):184–195. doi:10.1089/neu.2007.0438
de Castro RC Jr, Burns CL, McAdoo DJ, Romanic AM (2000) Metalloproteinase increases in the injured rat spinal cord. Neuroreport 11(16):3551–3554
Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD (1996) Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci U S A 93(9):3942–3946
Davies AL, Hayes KC, Dekaban GA (2007) Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil 88(11):1384–1393. doi:10.1016/j.apmr.2007.08.004
Leskovar A, Moriarty LJ, Turek JJ, Schoenlein IA, Borgens RB (2000) The macrophage in acute neural injury: changes in cell numbers over time and levels of cytokine production in mammalian central and peripheral nervous systems. J Exp Biol 203(Pt 12):1783–1795
Pan W, Kastin AJ (2001) Increase in TNFalpha transport after SCI is specific for time, region, and type of lesion. Exp Neurol 170(2):357–363. doi:10.1006/exnr.2001.7702
Lee YB, Yune TY, Baik SY, Shin YH, Du S, Rhim H, Lee EB, Kim YC et al (2000) Role of tumor necrosis factor-alpha in neuronal and glial apoptosis after spinal cord injury. Exp Neurol 166(1):190–195. doi:10.1006/exnr.2000.7494
Pan W, Banks WA, Kastin AJ (1997) Blood-brain barrier permeability to ebiratide and TNF in acute spinal cord injury. Exp Neurol 146(2):367–373. doi:10.1006/exnr.1997.6533
Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J et al (1999) Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma 16(10):851–863
Lotan M, Solomon A, Ben-Bassat S, Schwartz M (1994) Cytokines modulate the inflammatory response and change permissiveness to neuronal adhesion in injured mammalian central nervous system. Exp Neurol 126(2):284–290. doi:10.1006/exnr.1994.1066
Franzen R, Schoenen J, Leprince P, Joosten E, Moonen G, Martin D (1998) Effects of macrophage transplantation in the injured adult rat spinal cord: a combined immunocytochemical and biochemical study. J Neurosci Res 51(3):316–327
Li GL, Brodin G, Farooque M, Funa K, Holtz A, Wang WL, Olsson Y (1996) Apoptosis and expression of Bcl-2 after compression trauma to rat spinal cord. J Neuropathol Exp Neurol 55(3):280–289
Probert L, Selmaj K (1997) TNF and related molecules: trends in neuroscience and clinical applications. J Neuroimmunol 72(2):113–117
Probert L, Akassoglou K, Kassiotis G, Pasparakis M, Alexopoulou L, Kollias G (1997) TNF-alpha transgenic and knockout models of CNS inflammation and degeneration. J Neuroimmunol 72(2):137–141
Trickler WJ, Mayhan WG, Miller DW (2005) Brain microvessel endothelial cell responses to tumor necrosis factor-alpha involve a nuclear factor kappa B (NF-kappaB) signal transduction pathway. Brain Res 1048(1-2):24–31. doi:10.1016/j.brainres.2005.04.028
He F, Peng J, Deng XL, Yang LF, Camara AD, Omran A, Wang GL, Wu LW et al (2012) Mechanisms of tumor necrosis factor-alpha-induced leaks in intestine epithelial barrier. Cytokine 59(2):264–272. doi:10.1016/j.cyto.2012.04.008
Camussi G, Turello E, Bussolino F, Baglioni C (1991) Tumor necrosis factor alters cytoskeletal organization and barrier function of endothelial cells. Int Arch Allergy Appl Immunol 96(1):84–91
Kim KS, Wass CA, Cross AS, Opal SM (1992) Modulation of blood-brain barrier permeability by tumor necrosis factor and antibody to tumor necrosis factor in the rat. Lymphokine Cytokine Res 11(6):293–298
Duchini A, Govindarajan S, Santucci M, Zampi G, Hofman FM (1996) Effects of tumor necrosis factor-alpha and interleukin-6 on fluid-phase permeability and ammonia diffusion in CNS-derived endothelial cells. J Investig Med 44(8):474–482
Pan W, Kastin AJ (2002) TNFalpha transport across the blood-brain barrier is abolished in receptor knockout mice. Exp Neurol 174(2):193–200. doi:10.1006/exnr.2002.7871
Pan W, Csernus B, Kastin AJ (2003) Upregulation of p55 and p75 receptors mediating TNF-alpha transport across the injured blood-spinal cord barrier. J Mol Neurosci: MN 21(2):173–184. doi:10.1385/JMN:21:2:173
Pan W, Kastin AJ, Bell RL, Olson RD (1999) Upregulation of tumor necrosis factor alpha transport across the blood-brain barrier after acute compressive spinal cord injury. J Neurosci Off J Soc Neurosci 19(9):3649–3655
Kim GM, Xu J, Song SK, Yan P, Ku G, Xu XM, Hsu CY (2001) Tumor necrosis factor receptor deletion reduces nuclear factor-kappaB activation, cellular inhibitor of apoptosis protein 2 expression, and functional recovery after traumatic spinal cord injury. J Neurosci Off J Soc Neurosci 21(17):6617–6625
Kumar H, Lim HW, More SV, Kim BW, Koppula S, Kim IS, Choi DK (2012) The role of free radicals in the aging brain and Parkinson’s disease: convergence and parallelism. Int J Mol Sci 13(8):10478–10504. doi:10.3390/ijms130810478
McCoubrey WK Jr, Maines MD (1994) The structure, organization and differential expression of the gene encoding rat heme oxygenase-2. Gene 139(2):155–161
McCoubrey WK Jr, Huang TJ, Maines MD (1997) Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem/FEBS 247(2):725–732
McCoubrey WK Jr, Huang TJ, Maines MD (1997) Heme oxygenase-2 is a hemoprotein and binds heme through heme regulatory motifs that are not involved in heme catalysis. J Biol Chem 272(19):12568–12574
Panahian N, Maines MD (2001) Site of injury-directed induction of heme oxygenase-1 and -2 in experimental spinal cord injury: differential functions in neuronal defense mechanisms? J Neurochem 76(2):539–554
Mautes AE, Bergeron M, Sharp FR, Panter SS, Weinzierl M, Guenther K, Noble LJ (2000) Sustained induction of heme oxygenase-1 in the traumatized spinal cord. Exp Neurol 166(2):254–265. doi:10.1006/exnr.2000.7520
Liu Y, Tachibana T, Dai Y, Kondo E, Fukuoka T, Yamanaka H, Noguchi K (2002) Heme oxygenase-1 expression after spinal cord injury: the induction in activated neutrophils. J Neurotrauma 19(4):479–490. doi:10.1089/08977150252932424
Mautes AE, Kim DH, Sharp FR, Panter S, Sato M, Maida N, Bergeron M, Guenther K et al (1998) Induction of heme oxygenase-1 (HO-1) in the contused spinal cord of the rat. Brain Res 795(1-2):17–24
Lin Y, Vreman HJ, Wong RJ, Tjoa T, Yamauchi T, Noble-Haeusslein LJ (2007) Heme oxygenase-1 stabilizes the blood-spinal cord barrier and limits oxidative stress and white matter damage in the acutely injured murine spinal cord. J Cereb Blood Flow Metab 27(5):1010–1021. doi:10.1038/sj.jcbfm.9600412
Yamauchi T, Lin Y, Sharp FR, Noble-Haeusslein LJ (2004) Hemin induces heme oxygenase-1 in spinal cord vasculature and attenuates barrier disruption and neutrophil infiltration in the injured murine spinal cord. J Neurotrauma 21(8):1017–1030. doi:10.1089/0897715041651042
Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A (2004) Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol 165(3):1045–1053. doi:10.1016/S0002-9440(10)63365-2
Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y et al (1999) Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 103(1):129–135. doi:10.1172/JCI4165
Wagener FA, da Silva JL, Farley T, de Witte T, Kappas A, Abraham NG (1999) Differential effects of heme oxygenase isoforms on heme mediation of endothelial intracellular adhesion molecule 1 expression. J Pharmacol Exp Ther 291(1):416–423
Justicia C, Panes J, Sole S, Cervera A, Deulofeu R, Chamorro A, Planas AM (2003) Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab 23(12):1430–1440. doi:10.1097/01.WCB.0000090680.07515.C8
Lee TS, Chau LY (2002) Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med 8(3):240–246. doi:10.1038/nm0302-240
Mautes AE, Noble LJ (2000) Co-induction of HSP70 and heme oxygenase-1 in macrophages and glia after spinal cord contusion in the rat. Brain Res 883(2):233–237
Wang N, Wang G, Hao J, Ma J, Wang Y, Jiang X, Jiang H (2012) Curcumin ameliorates hydrogen peroxide-induced epithelial barrier disruption by upregulating heme oxygenase-1 expression in human intestinal epithelial cells. Dig Dis Sci 57(7):1792–1801. doi:10.1007/s10620-012-2094-7
Thomas M, Augustin HG (2009) The role of the Angiopoietins in vascular morphogenesis. Angiogenesis 12(2):125–137. doi:10.1007/s10456-009-9147-3
Hansen TM, Moss AJ, Brindle NP (2008) Vascular endothelial growth factor and angiopoietins in neurovascular regeneration and protection following stroke. Curr Neurovasc Res 5(4):236–245
Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, Feng Y, Gao Q et al (2007) Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab 27(10):1684–1691. doi:10.1038/sj.jcbfm.9600475
Kim H, Lee JM, Park JS, Jo SA, Kim YO, Kim CW, Jo I (2008) Dexamethasone coordinately regulates angiopoietin-1 and VEGF: a mechanism of glucocorticoid-induced stabilization of blood-brain barrier. Biochem Biophys Res Commun 372(1):243–248. doi:10.1016/j.bbrc.2008.05.025
Han S, Arnold SA, Sithu SD, Mahoney ET, Geralds JT, Tran P, Benton RL, Maddie MA et al (2010) Rescuing vasculature with intravenous angiopoietin-1 and alpha v beta 3 integrin peptide is protective after spinal cord injury. Brain J Neurol 133(Pt 4):1026–1042. doi:10.1093/brain/awq034
Ritz MF, Graumann U, Gutierrez B, Hausmann O (2010) Traumatic spinal cord injury alters angiogenic factors and TGF-beta1 that may affect vascular recovery. Curr Neurovasc Res 7(4):301–310
Herrera JJ, Sundberg LM, Zentilin L, Giacca M, Narayana PA (2010) Sustained expression of vascular endothelial growth factor and angiopoietin-1 improves blood-spinal cord barrier integrity and functional recovery after spinal cord injury. J Neurotrauma 27(11):2067–2076. doi:10.1089/neu.2010.1403
Valenzuela DM, Griffiths JA, Rojas J, Aldrich TH, Jones PF, Zhou H, McClain J, Copeland NG et al (1999) Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci U S A 96(5):1904–1909
Durham-Lee JC, Wu Y, Mokkapati VU, Paulucci-Holthauzen AA, Nesic O (2012) Induction of angiopoietin-2 after spinal cord injury. Neuroscience 202:454–464. doi:10.1016/j.neuroscience.2011.09.058
Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G et al (2000) Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res 87(7):603–607
Nourhaghighi N, Teichert-Kuliszewska K, Davis J, Stewart DJ, Nag S (2003) Altered expression of angiopoietins during blood-brain barrier breakdown and angiogenesis. Lab Invest 83(8):1211–1222
Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, Divoux D, Mackenzie ET et al (2005) VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab 25(11):1491–1504. doi:10.1038/sj.jcbfm.9600148
Nambu H, Nambu R, Oshima Y, Hackett SF, Okoye G, Wiegand S, Yancopoulos G, Zack DJ et al (2004) Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther 11(10):865–873. doi:10.1038/sj.gt.3302230
Stewart JM, Gera L, Chan DC, Whalley ET, Hanson WL, Zuzack JS (1997) Potent, long-acting bradykinin antagonists for a wide range of applications. Can J Physiol Pharmacol 75(6):719–724
Marmarou A, Nichols J, Burgess J, Newell D, Troha J, Burnham D, Pitts L (1999) Effects of the bradykinin antagonist Bradycor (deltibant, CP-1027) in severe traumatic brain injury: results of a multi-center, randomized, placebo-controlled trial. American Brain Inj Consortium Study Group J Neurotrauma 16(6):431–444
Narotam PK, Rodell TC, Nadvi SS, Bhoola KD, Troha JM, Parbhoosingh R, van Dellen JR (1998) Traumatic brain contusions: a clinical role for the kinin antagonist CP-0127. Acta Neurochir 140(8):793–802, discussion 802-793
Zausinger S, Lumenta DB, Pruneau D, Schmid-Elsaesser R, Plesnila N, Baethmann A (2002) Effects of LF 16-0687 Ms, a bradykinin B(2) receptor antagonist, on brain edema formation and tissue damage in a rat model of temporary focal cerebral ischemia. Brain Res 950(1-2):268–278
Sharma HS (2000) A bradykinin BK2 receptor antagonist HOE-140 attenuates blood-spinal cord barrier permeability following a focal trauma to the rat spinal cord. In: Brain Edema XI. Springer, Berlin, pp 159–163
Pan W, Kastin AJ, Gera L, Stewart JM (2001) Bradykinin antagonist decreases early disruption of the blood-spinal cord barrier after spinal cord injury in mice. Neurosci Lett 307(1):25–28
Yan-Feng W, Gang L, Yan-Ting G (2008) Bradykinin preconditioning induces protective effects on the spinal cord ischemic injury of rats. Neurosci Lett 433(2):114–118. doi:10.1016/j.neulet.2008.01.010
Mechirova E, Danielisova V, Domorakova I, Dankova M, Stebnicky M, Mickova H, Burda J (2014) Bradykinin preconditioning affects the number of degenerated neurons and the level of antioxidant enzymes in spinal cord ischemia in rabbits. Acta Histochem 116(1):252–257. doi:10.1016/j.acthis.2013.07.010
Laroux FS, Pavlick KP, Hines IN, Kawachi S, Harada H, Bharwani S, Hoffman JM, Grisham MB (2001) Role of nitric oxide in inflammation. Acta Physiol Scand 173(1):113–118. doi:10.1046/j.1365-201X.2001.00891.x
Knowles RG, Moncada S (1994) Nitric oxide synthases in mammals. Biochem J 298(Pt 2):249–258
Koppenol WH, Traynham JG (1996) Say NO to nitric oxide: nomenclature for nitrogen- and oxygen-containing compounds. Methods Enzymol 268:3–7
Bredt DS, Snyder SH (1994) Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem 63:175–195. doi:10.1146/annurev.bi.63.070194.001135
Xiong Y, Rabchevsky AG, Hall ED (2007) Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J Neurochem 100(3):639–649. doi:10.1111/j.1471-4159.2006.04312.x
Carrico KM, Vaishnav R, Hall ED (2009) Temporal and spatial dynamics of peroxynitrite-induced oxidative damage after spinal cord contusion injury. J Neurotrauma 26(8):1369–1378. doi:10.1089/neu.2008-0870
Marsala J, Kluchova D, Marsala M (1997) Spinal cord gray matter layers rich in NADPH diaphorase-positive neurons are refractory to ischemia-reperfusion-induced injury: a histochemical and silver impregnation study in rabbit. Exp Neurol 145(1):165–179. doi:10.1006/exnr.1997.6455
Hama AT, Sagen J (1994) Induction of spinal NADPH-diaphorase by nerve injury is attenuated by adrenal medullary transplants. Brain Res 640(1-2):345–351
Vincent SR (1994) Nitric oxide: a radical neurotransmitter in the central nervous system. Prog Neurobiol 42(1):129–160
Liu D, Ling X, Wen J, Liu J (2000) The role of reactive nitrogen species in secondary spinal cord injury: formation of nitric oxide, peroxynitrite, and nitrated protein. J Neurochem 75(5):2144–2154
Hamada Y, Ikata T, Katoh S, Tsuchiya K, Niwa M, Tsutsumishita Y, Fukuzawa K (1996) Roles of nitric oxide in compression injury of rat spinal cord. Free Radic Biol Med 20(1):1–9
Nakahara S, Yone K, Setoguchi T, Yamaura I, Arishima Y, Yoshino S, Komiya S (2002) Changes in nitric oxide and expression of nitric oxide synthase in spinal cord after acute traumatic injury in rats. J Neurotrauma 19(11):1467–1474. doi:10.1089/089771502320914697
Sharma HS, Nyberg F, Westman J, Alm P, Gordh T, Lindholm D (1998) Brain derived neurotrophic factor and insulin like growth factor-1 attenuate upregulation of nitric oxide synthase and cell injury following trauma to the spinal cord. An immunohistochemical study in the rat. Amino Acids 14(1-3):121–129
Sharma HS (2010) A combination of tumor necrosis factor-alpha and neuronal nitric oxide synthase antibodies applied topically over the traumatized spinal cord enhances neuroprotection and functional recovery in the rat. Ann N Y Acad Sci 1199:175–185. doi:10.1111/j.1749-6632.2009.05327.x
Pearse DD, Chatzipanteli K, Marcillo AE, Bunge MB, Dietrich WD (2003) Comparison of iNOS inhibition by antisense and pharmacological inhibitors after spinal cord injury. J Neuropathol Exp Neurol 62(11):1096–1107
Maggio DM, Chatzipanteli K, Masters N, Patel SP, Dietrich WD, Pearse DD (2012) Acute molecular perturbation of inducible nitric oxide synthase with an antisense approach enhances neuronal preservation and functional recovery after contusive spinal cord injury. J Neurotrauma 29(12):2244–2249. doi:10.1089/neu.2012.2371
Zimmermann M (1997) Endothelin in cerebral vasospasm. Clinical and experimental results. J Neurosurg Sci 41(2):139–151
Kallakuri S, Kreipke CW, Schafer PC, Schafer SM, Rafols JA (2010) Brain cellular localization of endothelin receptors A and B in a rodent model of diffuse traumatic brain injury. Neuroscience 168(3):820–830. doi:10.1016/j.neuroscience.2010.01.018
Barnes K, Turner AJ (1997) The endothelin system and endothelin-converting enzyme in the brain: molecular and cellular studies. Neurochem Res 22(8):1033–1040
Dehouck MP, Vigne P, Torpier G, Breittmayer JP, Cecchelli R, Frelin C (1997) Endothelin-1 as a mediator of endothelial cell-pericyte interactions in bovine brain capillaries. J Cereb Blood Flow Metab 17(4):464–469. doi:10.1097/00004647-199704000-00012
Hama H, Kasuya Y, Sakurai T, Yamada G, Suzuki N, Masaki T, Goto K (1997) Role of endothelin-1 in astrocyte responses after acute brain damage. J Neurosci Res 47(6):590–602
McKenzie AL, Hall JJ, Aihara N, Fukuda K, Noble LJ (1995) Immunolocalization of endothelin in the traumatized spinal cord: relationship to blood-spinal cord barrier breakdown. J Neurotrauma 12(3):257–268
Westmark R, Noble LJ, Fukuda K, Aihara N, McKenzie AL (1995) Intrathecal administration of endothelin-1 in the rat: impact on spinal cord blood flow and the blood-spinal cord barrier. Neurosci Lett 192(3):173–176
Giaid A, Gibson SJ, Ibrahim BN, Legon S, Bloom SR, Yanagisawa M, Masaki T, Varndell IM et al (1989) Endothelin 1, an endothelium-derived peptide, is expressed in neurons of the human spinal cord and dorsal root ganglia. Proc Natl Acad Sci U S A 86(19):7634–7638
Peters CM, Rogers SD, Pomonis JD, Egnaczyk GF, Keyser CP, Schmidt JA, Ghilardi JR, Maggio JE et al (2003) Endothelin receptor expression in the normal and injured spinal cord: potential involvement in injury-induced ischemia and gliosis. Exp Neurol 180(1):1–13
Siren AL, Knerlich F, Schilling L, Kamrowski-Kruck H, Hahn A, Ehrenreich H (2000) Differential glial and vascular expression of endothelins and their receptors in rat brain after neurotrauma. Neurochem Res 25(7):957–969
MacCumber MW, Ross CA, Snyder SH (1990) Endothelin in brain: receptors, mitogenesis, and biosynthesis in glial cells. Proc Natl Acad Sci U S A 87(6):2359–2363
Bertsch T, Kuehl S, Muehlhauser F, Walter S, Hodapp B, Rossol S, Schmeck J, Ragoschke A et al (2001) Source of endothelin-1 in subarachnoid hemorrhage. Clin Chem Lab Med: CCLM/FESCC 39(4):341–345. doi:10.1515/CCLM.2001.053
Yamashita K, Kataoka Y, Sakurai-Yamashita Y, Shigematsu K, Himeno A, Niwa M, Taniyama K (2000) Involvement of glial endothelin/nitric oxide in delayed neuronal death of rat hippocampus after transient forebrain ischemia. Cell Mol Neurobiol 20(5):541–551
Salzman SK, Acosta R, Beck G, Madden J, Boxer B, Ohlstein EH (1996) Spinal endothelin content is elevated after moderate local trauma in the rat to levels associated with locomotor dysfunction after intrathecal injection. J Neurotrauma 13(2):93–101
Uesugi M, Kasuya Y, Hayashi K, Goto K (1998) SB209670, a potent endothelin receptor antagonist, prevents or delays axonal degeneration after spinal cord injury. Brain Res 786(1-2):235–239
Weinzierl M, Mautes AE, Whetstone W, Lin Y, Noble-Haeusslein LJ (2004) Endothelin-mediated induction of heme oxygenase-1 in the spinal cord is attenuated in transgenic mice overexpressing superoxide dismutase. Brain Res 1030(1):125–132. doi:10.1016/j.brainres.2004.09.060
Guo J, Li Y, He Z, Zhang B, Li Y, Hu J, Han M, Xu Y et al (2014) Targeting endothelin receptors A and B attenuates the inflammatory response and improves locomotor function following spinal cord injury in mice. Int J Mol Med 34(1):74–82
Lee JY, Kim HS, Choi HY, Oh TH, Ju BG, Yune TY (2012) Valproic acid attenuates blood-spinal cord barrier disruption by inhibiting matrix metalloprotease-9 activity and improves functional recovery after spinal cord injury. J Neurochem 121(5):818–829. doi:10.1111/j.1471-4159.2012.07731.x
Fang B, Li XQ, Bi B, Tan WF, Liu G, Zhang Y, Ma H (2015) Dexmedetomidine attenuates blood-spinal cord barrier disruption induced by spinal cord ischemia reperfusion injury in rats. Cell Physiol Biochem 36(1):373–383. doi:10.1159/000430107
Sharma HS, Badgaiyan RD, Alm P, Mohanty S, Wiklund L (2005) Neuroprotective effects of nitric oxide synthase inhibitors in spinal cord injury-induced pathophysiology and motor functions: an experimental study in the rat. Ann N Y Acad Sci 1053:422–434. doi:10.1196/annals.1344.037
Lee JY, Choi HY, Na WH, Ju BG, Yune TY (2014) Ghrelin inhibits BSCB disruption/hemorrhage by attenuating MMP-9 and SUR1/TrpM4 expression and activation after spinal cord injury. Biochim Biophys Acta 1842(12 Pt A):2403–2412. doi:10.1016/j.bbadis.2014.09.006
Tian DS, Liu JL, Xie MJ, Zhan Y, Qu WS, Yu ZY, Tang ZP, Pan DJ et al (2009) Tamoxifen attenuates inflammatory-mediated damage and improves functional outcome after spinal cord injury in rats. J Neurochem 109(6):1658–1667. doi:10.1111/j.1471-4159.2009.06077.x
Repici M, Chen X, Morel MP, Doulazmi M, Sclip A, Cannaya V, Veglianese P, Kraftsik R et al (2012) Specific inhibition of the JNK pathway promotes locomotor recovery and neuroprotection after mouse spinal cord injury. Neurobiol Dis 46(3):710–721. doi:10.1016/j.nbd.2012.03.014
Tonai T, Shiba K, Taketani Y, Ohmoto Y, Murata K, Muraguchi M, Ohsaki H, Takeda E et al (2001) A neutrophil elastase inhibitor (ONO-5046) reduces neurologic damage after spinal cord injury in rats. J Neurochem 78(5):1064–1072
Fan Z, Cao Y, Zhang Z, Wang Y, Yu D, Zhang M, Mei X, Lu G (2012) Effect of aminoguanidine on spinal cord edema of acute spinal cord injury in rats. Zhongguo xiu fu chong jian wai ke za zhi = Zhongguo xiufu chongjian waike zazhi = Chinese journal of reparative and reconstructive surgery 26(8):984–988
Fang B, Wang H, Sun XJ, Li XQ, Ai CY, Tan WF, White PF, Ma H (2013) Intrathecal transplantation of bone marrow stromal cells attenuates blood-spinal cord barrier disruption induced by spinal cord ischemia-reperfusion injury in rabbits. J Vasc Surg 58(4):1043–1052. doi:10.1016/j.jvs.2012.11.087
Fang B, Li XM, Sun XJ, Bao NR, Ren XY, Lv HW, Ma H (2013) Ischemic preconditioning protects against spinal cord ischemia-reperfusion injury in rabbits by Attenuating Blood Spinal Cord barrier disruption. Int J Mol Sci 14(5):10343–10354. doi:10.3390/ijms140510343
Lee JY, Choi HY, Na WH, Ju BG, Yune TY (2015) 17beta-Estradiol inhibits MMP-9 and SUR1/TrpM4 expression and activation and thereby attenuates bscb disruption/hemorrhage after spinal cord injury in male rats. Endocrinology 156(5):1838–1850. doi:10.1210/en.2014-1832
Wu Q, Jing Y, Yuan X, Zhang X, Li B, Liu M, Wang B, Li H et al (2014) Melatonin treatment protects against acute spinal cord injury-induced disruption of blood spinal cord barrier in mice. J Mol Neurosci: MN 54(4):714–722. doi:10.1007/s12031-014-0430-4
Li XQ, Cao XZ, Wang J, Fang B, Tan WF, Ma H (2014) Sevoflurane preconditioning ameliorates neuronal deficits by inhibiting microglial MMP-9 expression after spinal cord ischemia/reperfusion in rats. Mol Brain 7:69. doi:10.1186/s13041-014-0069-7
Li Y-Q, Ballinger JR, Nordal RA, Su Z-F, Wong CS (2001) Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res 61(8):3348–3354
Kiyatkin EA, Sharma HS (2015) Not just the brain: methamphetamine disrupts blood-spinal cord barrier and induces acute glial activation and structural damage of spinal cord cells. CNS Neurol Disord Drug Targets 14(2):282–294
Nyberg F, Sharma HS (2002) Repeated topical application of growth hormone attenuates blood-spinal cord barrier permeability and edema formation following spinal cord injury: an experimental study in the rat using Evans blue, ([125])I-sodium and lanthanum tracers. Amino Acids 23(1-3):231–239. doi:10.1007/s00726-001-0134-2
Matsushita T, Lankford KL, Arroyo EJ, Sasaki M, Neyazi M, Radtke C, Kocsis JD (2015) Diffuse and persistent blood-spinal cord barrier disruption after contusive spinal cord injury rapidly recovers following intravenous infusion of bone marrow mesenchymal stem cells. Exp Neurol 267:152–164. doi:10.1016/j.expneurol.2015.03.001
Acknowledgments
This work was supported by a grant of the National Research Foundation of Korea (NRF) (NRF-2014R1A1A2059118) and the Ministry of Science, ICT and Future Planning (NRF-2013R1A2A1A09013980).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kumar, H., Ropper, A.E., Lee, SH. et al. Propitious Therapeutic Modulators to Prevent Blood-Spinal Cord Barrier Disruption in Spinal Cord Injury. Mol Neurobiol 54, 3578–3590 (2017). https://doi.org/10.1007/s12035-016-9910-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9910-6