Abstract
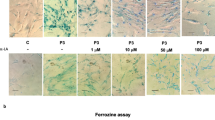
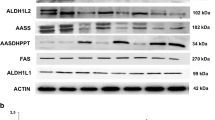
Neurodegeneration with brain iron accumulation (NBIA) is a group of inherited neurologic disorders in which iron accumulates in the basal ganglia resulting in progressive dystonia, spasticity, parkinsonism, neuropsychiatric abnormalities, and optic atrophy or retinal degeneration. The most prevalent form of NBIA is pantothenate kinase-associated neurodegeneration (PKAN) associated with mutations in the gene of pantothenate kinase 2 (PANK2), which is essential for coenzyme A (CoA) synthesis. There is no cure for NBIA nor is there a standard course of treatment. In the current work, we describe that fibroblasts derived from patients harbouring PANK2 mutations can reproduce many of the cellular pathological alterations found in the disease, such as intracellular iron and lipofuscin accumulation, increased oxidative stress, and mitochondrial dysfunction. Furthermore, mutant fibroblasts showed a characteristic senescent morphology. Treatment with pantothenate, the PANK2 enzyme substrate, was able to correct all pathological alterations in responder mutant fibroblasts with residual PANK2 enzyme expression. However, pantothenate had no effect on mutant fibroblasts with truncated/incomplete protein expression. The positive effect of pantothenate in particular mutations was also confirmed in induced neurons obtained by direct reprograming of mutant fibroblasts. Our results suggest that pantothenate treatment can stabilize the expression levels of PANK2 in selected mutations. These results encourage us to propose our screening model as a quick and easy way to detect pantothenate-responder patients with PANK2 mutations. The existence of residual enzyme expression in some affected individuals raises the possibility of treatment using high dose of pantothenate.









Similar content being viewed by others
References
Gregory A, Polster BJ, Hayflick SJ (2009) Clinical and genetic delineation of neurodegeneration with brain iron accumulation. J Med Genet 46(2):73–80
Hayflick SJ, Westaway SK, Levinson B, Zhou B, Johnson MA, Ching KH, Gitschier J (2003) Genetic, clinical, and radiographic delineation of Hallervorden-Spatz syndrome. N Engl J Med 348(1):33–40
Di Meo I, Tiranti V (2018) Classification and molecular pathogenesis of NBIA syndromes. Eur J Paediatr Neurol 22(2):272–284. https://doi.org/10.1016/j.ejpn.2018.01.008
Arber CE, Li A, Houlden H, Wray S (2016) Review: insights into molecular mechanisms of disease in neurodegeneration with brain iron accumulation: unifying theories. Neuropathol Appl Neurobiol 42(3):220–241. https://doi.org/10.1111/nan.12242
Levi S, Finazzi D (2014) Neurodegeneration with brain iron accumulation: update on pathogenic mechanisms. Front Pharmacol 5:99
Brunetti D, Dusi S, Morbin M, Uggetti A, Moda F, D'Amato I, Giordano C, d'Amati G et al (2012) Pantothenate kinase-associated neurodegeneration: altered mitochondria membrane potential and defective respiration in Pank2 knock-out mouse model. Hum Mol Genet 21(24):5294–5305. https://doi.org/10.1093/hmg/dds380
Gregory A, Hayflick SJ (2005) Neurodegeneration with brain iron accumulation. Folia Neuropathologica/Association of Polish Neuropathologists and Medical Research Centre. Folia Neuropathol 43(4):286–296
Afshar K, Gonczy P, DiNardo S, Wasserman SA (2001) Fumble encodes a pantothenate kinase homolog required for proper mitosis and meiosis in Drosophila melanogaster. Genetics 157(3):1267–1276
Leonardi R, Zhang YM, Lykidis A, Rock CO, Jackowski S (2007) Localization and regulation of mouse pantothenate kinase 2. FEBS Lett 581(24):4639–4644
Kuo YM, Duncan JL, Westaway SK, Yang H, Nune G, Xu EY, Hayflick SJ, Gitschier J (2005) Deficiency of pantothenate kinase 2 (Pank2) in mice leads to retinal degeneration and azoospermia. Hum Mol Genet 14(1):49–57
Kuo YM, Hayflick SJ, Gitschier J (2007) Deprivation of pantothenic acid elicits a movement disorder and azoospermia in a mouse model of pantothenate kinase-associated neurodegeneration. J Inherit Metab Dis 30(3):310–317
Bosveld F, Rana A, van der Wouden PE, Lemstra W, Ritsema M, Kampinga HH, Sibon OC (2008) De novo CoA biosynthesis is required to maintain DNA integrity during development of the Drosophila nervous system. Hum Mol Genet 17(13):2058–2069
Rana A, Seinen E, Siudeja K, Muntendam R, Srinivasan B, van der Want JJ, Hayflick S, Reijngoud DJ et al (2010) Pantethine rescues a Drosophila model for pantothenate kinase-associated neurodegeneration. Proc Natl Acad Sci U S A 107(15):6988–6993
Campanella A, Privitera D, Guaraldo M, Rovelli E, Barzaghi C, Garavaglia B, Santambrogio P, Cozzi A et al (2012) Skin fibroblasts from pantothenate kinase-associated neurodegeneration patients show altered cellular oxidative status and have defective iron-handling properties. Hum Mol Genet 21(18):4049–4059. https://doi.org/10.1093/hmg/dds229
Adzhubei I, Jordan DM, Sunyaev SR (2013) Predicting functional effect of human missense mutations using PolyPhen-2. Current protocols in human genetics/editorial board, Jonathan L Haines [et al. Chapter 7:Unit7 20. https://doi.org/10.1002/0471142905.hg0720s76
Zhou B, Westaway SK, Levinson B, Johnson MA, Gitschier J, Hayflick SJ (2001) A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat Genet 28(4):345–349
Dang TN, Bishop GM, Dringen R, Robinson SR (2010) The putative heme transporter HCP1 is expressed in cultured astrocytes and contributes to the uptake of hemin. Glia 58(1):55–65
Georgakopoulou EA, Tsimaratou K, Evangelou K, Fernandez Marcos PJ, Zoumpourlis V, Trougakos IP, Kletsas D, Bartek J et al (2013) Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging 5(1):37–50
Boulton M, Marshall J (1985) Repigmentation of human retinal pigment epithelial cells in vitro. Exp Eye Res 41(2):209–218
Biesemeier A, Schraermeyer U, Eibl O (2011) Quantitative chemical analysis of ocular melanosomes in stained and non-stained tissues. Micron 42(5):461–470. https://doi.org/10.1016/j.micron.2011.01.004
Riemer J, Hoepken HH, Czerwinska H, Robinson SR, Dringen R (2004) Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal Biochem 331(2):370–375. https://doi.org/10.1016/j.ab.2004.03.049
Tarohda T, Ishida Y, Kawai K, Yamamoto M, Amano R (2005) Regional distributions of manganese, iron, copper, and zinc in the brains of 6-hydroxydopamine-induced parkinsonian rats. Anal Bioanal Chem 383(2):224–234. https://doi.org/10.1007/s00216-005-3423-x
Shibata K, Nakai T, Fukuwatari T (2012) Simultaneous high-performance liquid chromatography determination of coenzyme A, dephospho-coenzyme A, and acetyl-coenzyme A in normal and pantothenic acid-deficient rats. Anal Biochem 430(2):151–155. https://doi.org/10.1016/j.ab.2012.08.010
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275
Santambrogio P, Erba BG, Campanella A, Cozzi A, Causarano V, Cremonesi L, Galli A, Della Porta MG et al (2011) Over-expression of mitochondrial ferritin affects the JAK2/STAT5 pathway in K562 cells and causes mitochondrial iron accumulation. Haematologica 96(10):1424–1432. https://doi.org/10.3324/haematol.2011.042952
Rodriguez-Arribas M, Pizarro-Estrella E, Gomez-Sanchez R, Yakhine-Diop SM, Gragera-Hidalgo A, Cristo A, Bravo-San Pedro JM, Gonzalez-Polo RA et al (2016) IFDOTMETER: a new software application for automated immunofluorescence analysis. J Lab Autom 21(2):246–259. https://doi.org/10.1177/2211068215600650
Drouin-Ouellet J, Lau S, Brattas PL, Rylander Ottosson D, Pircs K, Grassi DA, Collins LM, Vuono R et al (2017) REST suppression mediates neural conversion of adult human fibroblasts via microRNA-dependent and -independent pathways. EMBO Mol Med 9(8):1117–1131. https://doi.org/10.15252/emmm.201607471
Shrigley S, Pircs K, Barker RA, Parmar M, Drouin-Ouellet J (2018) Simple generation of a high yield culture of induced neurons from human adult skin fibroblasts. J Vis Exp 132. https://doi.org/10.3791/56904
Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D (1997) Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 15(9):871–875. https://doi.org/10.1038/nbt0997-871
Luckenbach MW, Green WR, Miller NR, Moser HW, Clark AW, Tennekoon G (1983) Ocular clinicopathologic correlation of Hallervorden-Spatz syndrome with acanthocytosis and pigmentary retinopathy. Am J Ophthalmol 95(3):369–382
Swaiman KF, Smith SA, Trock GL, Siddiqui AR (1983) Sea-blue histiocytes, lymphocytic cytosomes, movement disorder and 59Fe-uptake in basal ganglia: Hallervorden-Spatz disease or ceroid storage disease with abnormal isotope scan? Neurology 33(3):301–305
Defendini R, Markesbery WR, Mastri AR, Duffy PE (1973) Hallervorden-Spatz disease and infantile neuroaxonal dystrophy. Ultrastructural observations, anatomical pathology and nosology. J Neurol Sci 20(1):7–23
Park BE, Netsky MG, Betsill WL Jr (1975) Pathogenesis of pigment and spheroid formation in Hallervorden-Spatz syndrome and related disorders. Neurology 25(12):1172–1178
Bindewald-Wittich A, Han M, Schmitz-Valckenberg S, Snyder SR, Giese G, Bille JF, Holz FG (2006) Two-photon-excited fluorescence imaging of human RPE cells with a femtosecond Ti:sapphire laser. Invest Ophthalmol Vis Sci 47(10):4553–4557. https://doi.org/10.1167/iovs.05-1562
Newbury DE, Ritchie NW (2015) Performing elemental microanalysis with high accuracy and high precision by scanning electron microscopy/silicon drift detector energy-dispersive X-ray spectrometry (SEM/SDD-EDS). J Mater Sci 50(2):493–518. https://doi.org/10.1007/s10853-014-8685-2
Shioji K, Oyama Y, Okuma K, Nakagawa H (2010) Synthesis and properties of fluorescence probe for detection of peroxides in mitochondria. Bioorg Med Chem Lett 20(13):3911–3915. https://doi.org/10.1016/j.bmcl.2010.05.017
Orellana DI, Santambrogio P, Rubio A, Yekhlef L, Cancellieri C, Dusi S, Giannelli SG, Venco P et al (2016) Coenzyme A corrects pathological defects in human neurons of PANK2-associated neurodegeneration. EMBO Mol Med 8(10):1197–1211. https://doi.org/10.15252/emmm.201606391
Santambrogio P, Dusi S, Guaraldo M, Rotundo LI, Broccoli V, Garavaglia B, Tiranti V, Levi S (2015) Mitochondrial iron and energetic dysfunction distinguish fibroblasts and induced neurons from pantothenate kinase-associated neurodegeneration patients. Neurobiol Dis 81:144–153. https://doi.org/10.1016/j.nbd.2015.02.030
Ingrassia R, Memo M, Garavaglia B (2017) Ferrous iron up-regulation in fibroblasts of patients with beta propeller protein-associated neurodegeneration (BPAN). Front Genet 8:18. https://doi.org/10.3389/fgene.2017.00018
Nunez MT, Urrutia P, Mena N, Aguirre P, Tapia V, Salazar J (2012) Iron toxicity in neurodegeneration. Biometals 25(4):761–776. https://doi.org/10.1007/s10534-012-9523-0
Lan AP, Chen J, Chai ZF, Hu Y (2016) The neurotoxicity of iron, copper and cobalt in Parkinson’s disease through ROS-mediated mechanisms. Biometals 29(4):665–678. https://doi.org/10.1007/s10534-016-9942-4
Salvador GA, Uranga RM, Giusto NM (2010) Iron and mechanisms of neurotoxicity. Int J Alzheimers Dis 2011:720658–720659. https://doi.org/10.4061/2011/720658
Kruer MC (2013) The neuropathology of neurodegeneration with brain iron accumulation. Int Rev Neurobiol 110:165–194. https://doi.org/10.1016/B978-0-12-410502-7.00009-0
Matsunaga T, Kotamraju S, Kalivendi SV, Dhanasekaran A, Joseph J, Kalyanaraman B (2004) Ceramide-induced intracellular oxidant formation, iron signaling, and apoptosis in endothelial cells: protective role of endogenous nitric oxide. J Biol Chem 279(27):28614–28624. https://doi.org/10.1074/jbc.M400977200
Double KL, Dedov VN, Fedorow H, Kettle E, Halliday GM, Garner B, Brunk UT (2008) The comparative biology of neuromelanin and lipofuscin in the human brain. Cell Mol Life Sci 65(11):1669–1682. https://doi.org/10.1007/s00018-008-7581-9
Jolly RD, Douglas BV, Davey PM, Roiri JE (1995) Lipofuscin in bovine muscle and brain: a model for studying age pigment. Gerontology 41(Suppl 2):283–295
Jung T, Bader N, Grune T (2007) Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci 1119:97–111. https://doi.org/10.1196/annals.1404.008
Konig J, Ott C, Hugo M, Jung T, Bulteau AL, Grune T, Hohn A (2017) Mitochondrial contribution to lipofuscin formation. Redox Biol 11:673–681. https://doi.org/10.1016/j.redox.2017.01.017
Frolova MS, Surin AM, Braslavski AV, Vekshin NL (2015) Degradation of mitochondria to lipofuscin upon heating and illumination. Biofizika 60(6):1125–1131
Brunk UT, Terman A (2002) Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med 33(5):611–619
Powell SR, Wang P, Divald A, Teichberg S, Haridas V, McCloskey TW, Davies KJ, Katzeff H (2005) Aggregates of oxidized proteins (lipofuscin) induce apoptosis through proteasome inhibition and dysregulation of proapoptotic proteins. Free Radic Biol Med 38(8):1093–1101. https://doi.org/10.1016/j.freeradbiomed.2005.01.003
Hohn A, Grune T (2013) Lipofuscin: formation, effects and role of macroautophagy. Redox Biol 1:140–144. https://doi.org/10.1016/j.redox.2013.01.006
Kurz T, Terman A, Gustafsson B, Brunk UT (2008) Lysosomes and oxidative stress in aging and apoptosis. Biochim Biophys Acta 1780(11):1291–1303. https://doi.org/10.1016/j.bbagen.2008.01.009
Reeg S, Grune T (2015) Protein oxidation in aging: does it play a role in aging progression? Antioxid Redox Signal 23(3):239–255. https://doi.org/10.1089/ars.2014.6062
Lill R, Srinivasan V, Muhlenhoff U (2014) The role of mitochondria in cytosolic-nuclear iron-sulfur protein biogenesis and in cellular iron regulation. Curr Opin Microbiol 22:111–119. https://doi.org/10.1016/j.mib.2014.09.015
Lu C, Cortopassi G (2007) Frataxin knockdown causes loss of cytoplasmic iron-sulfur cluster functions, redox alterations and induction of heme transcripts. Arch Biochem Biophys 457(1):111–122. https://doi.org/10.1016/j.abb.2006.09.010
Poli M, Derosas M, Luscieti S, Cavadini P, Campanella A, Verardi R, Finazzi D, Arosio P. Pantothenate kinase-2 (Pank2) silencing causes cell growth reduction, cell-specific ferroportin upregulation and iron deregulation. Neurobiol Dis. 2010 Aug 39(2):204–10. https://doi.org/10.1016/j.nbd.2010.04.009. Epub 2010 Apr 23
Huang ML, Lane DJ, Richardson DR (2011) Mitochondrial mayhem: the mitochondrion as a modulator of iron metabolism and its role in disease. Antioxid Redox Signal 15(12):3003–3019. https://doi.org/10.1089/ars.2011.3921
Leonardi R, Zhang YM, Rock CO, Jackowski S (2005) Coenzyme A: back in action. Prog Lipid Res 44(2–3):125–153. https://doi.org/10.1016/j.plipres.2005.04.001
Leonardi R, Jackowski S (2007) Biosynthesis of pantothenic acid and coenzyme A. EcoSal Plus 2(2). https://doi.org/10.1128/ecosalplus.3.6.3.4
Garcia M, Leonardi R, Zhang YM, Rehg JE, Jackowski S (2012) Germline deletion of pantothenate kinases 1 and 2 reveals the key roles for CoA in postnatal metabolism. PLoS One 7(7):e40871. https://doi.org/10.1371/journal.pone.0040871
Acknowledgements
We thank María Pilar Burgos Domenech from IRNAS (Instituto de Recursos Naturales y Agrobiología de Sevilla) for her help with the ICP-MS assays and Carmen Jiménez de Haro from Instituto de Ciencia de Materiales de Sevilla (ICMS-US-CSIC) for her help with the SEM/EDX assays. We also thank Drs. Javier Abril Jaramillo, Anabel Vintimilla, Luis González Gutiérrez Solana, Pablo Mir, Marcos Madruga, Silvia Jesús, and Isidoro Caraballo for their support to the project.
Funding
This work was supported by FIS PI16/00786 grant, Instituto de Salud Carlos III, Spain and Fondo Europeo de Desarrollo Regional (FEDER-Unión Europea), Proyectos de Investigación de Excelencia de la Junta de Andalucía CTS-5725 and BIO-122, DGICYT BFU2015-64536-R, and by AEPMI (Asociación de Enfermos de Patología Mitocondrial) and ENACH (Asociación de Enfermos de Neurodegeneración con Acumulación Cerebral de Hierro).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(PDF 2105 kb)
Rights and permissions
About this article
Cite this article
Álvarez-Córdoba, M., Fernández Khoury, A., Villanueva-Paz, M. et al. Pantothenate Rescues Iron Accumulation in Pantothenate Kinase-Associated Neurodegeneration Depending on the Type of Mutation. Mol Neurobiol 56, 3638–3656 (2019). https://doi.org/10.1007/s12035-018-1333-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1333-0