Abstract
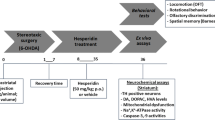
Flavonoids are considered therapeutic agents in neurodegenerative disease because of their neuroprotective activity. This study investigated the neuroprotective effects of hesperetin in the brains of mice administered hesperetin at 10 or 50 mg/kg body weight (BW) for five weeks. Hesperetin inhibited biomarkers of oxidative stress, such as the level of thiobarbituric acid-reactive substance (TBARS) and carbonyl content, although there was a significant reduction at the higher dose of hesperetin. Moreover, at the higher dose, hesperetin significantly activated the catalase and total superoxide dismutase (SOD) activities. The same patterns were observed in the protein expression, and the expression of CuZn-SOD was more pronounced than that of Mn-SOD. The reduced glutathione (GSH)/oxidized glutathione (GSSG) ratio was increased significantly in a dose-dependent manner, as well as the glutathione peroxidase (GSH-px) and glutathione reductase (GR) activities. Moreover, hesperetin did not induce apoptosis, even at the higher dose, as evidenced by caspase-3 expression and its activity. Based on these results, hesperetin may have a neuroprotective effect via the inhibition of oxidative damage, together with activation of the antioxidant enzyme system.
Similar content being viewed by others
References
Adams, J. D. Jr., Klaidman, L. K., Odunze, I. N., Shen, H. C., and Miller, C. A., Alzheimer’s and Parkinson’s disease. Brain levels of glutathione, glutathione disulfide, and vitamin E. Mol. Chem. Neuropathol., 14, 213–226 (1991).
Aebi, H., Catalase in vitro. Methods Enzymol., 105, 121–126 (1984).
Aldini, G., Dalle-Donne, I., Facino, R. M., Milzani, A., and Carini, M., Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med. Res. Rev., 27, 817–868 (2007).
Ansari, M. A., Joshi, G., Huang, Q., Opii, W. O., Abdul, H. M., Sultana, R., and Butterfield, D. A., In vivo administration of D609 leads to protection of subsequently isolated gerbil brain mitochondria subjected to in vitro oxidative stress induced by amyloid beta-peptide and other oxidative stressors: relevance to Alzheimer’s disease and other oxidative stress-related neurodegenerative disorders. Free Radic. Biol. Med., 41, 1694–1703 (2006).
Bharath, S., Hsu, M., Kaur, D., Rajagopalan, S., and Andersen, J. K., Glutathione, iron and Parkinson’s disease. Biochem. Pharmacol., 64, 1037–1048 (2002).
Bonilla, E., Huntington disease. Invest. Clin., 41, 117–141 (2000).
Carlberg, I. and Mannervik, B., Glutathione reductase. Methods Enzymol., 113, 484–490 (1985).
Cho, J., Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch. Pharm. Res., 29, 699–706 (2006).
Choi, E. J., Kim, G. D., Chee, K. M., and Kim, G. H., Effects of hesperetin on vessel structure formation in mouse embryonic stem (mES) cells. Nutrition, 22, 947–951 (2006).
Choi, E. J., Antioxidative effects of hesperetin against 7,12-dimethylbenz(a)anthracene-induced oxidative stress in mice. Life Sci., 82, 1059–1064 (2008).
Cooper, A. J. L., Glutathione in the brain: disorders of glutathione metabolism: The Molecular and Genetic Basis of Neurological Disease. Butterworth-Heinemann, Boston, 1997, pp. 1195–1230, (1997).
Cruz-Aguado, R., Turner, L. F., Diaz, C. M., and Pinero, J., Nerve growth factor and striatal glutathione metabolism in a rat model of Huntington’s disease. Restor. Neurol. Neurosci., 17, 217–221 (2000).
Erdem, E., Carlier, R., Idir, A. B., Masnou, P. O., Moulonguet, A., Adams, D., and Doyon, D., Gadolinium-enhanced MRI in central nervous system Behçet’s disease. Neuroradiology, 35, 142–144 (1993).
Flohé, L. and Günzler, W. A., Assays of glutathione peroxidase. Methods Enzymol., 105, 114–121 (1984).
Fridovich, I., Superoxide anion radical (O2−.), superoxide dismutases, and related matters. J. Biol. Chem., 272, 18515–18517 (1997).
Fridovich, I., Superoxide radical and superoxide dismutases. Annu. Rev. Biochem., 64, 97–112 (1995).
Garg, A., Garg, S., Zaneveld, L. J., and Singla, A. K., Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother. Res., 15, 655–669 (2001).
Hirrlinger, J., Schulz, J. B., and Dringen, R., Effects of dopamine on the glutathione metabolism of cultured astroglial cells: implications for Parkinson’s disease. J. Neurochem., 82, 458–467 (2002).
Hissin, P. J. and Hilf, R., A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem., 74, 214–226 (1976).
Ho, Y. S., Magnenat, J. L., Bronson, R. T., Cao, J., Gargano, M., Sugawara, M., and Funk, C. D., Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J. Biol. Chem., 272, 16644–16651 (1997a).
Ho, Y. S., Swenson, L., Derewenda, U., Serre, L., Wei, Y., Dauter, Z., Hattori, M., Adachi, T., Aoki, J., Arai, H., Inoue, K., and Derewenda, Z. S., Brain acetylhydrolase that inactivates platelet-activating factor is a G-protein-like trimer. Nature, 385, 89–93 (1997b).
Hwang, S. L. and Yen, G. C., Neuroprotective effects of the citrus flavanones against H2O2-induced cytotoxicity in PC12 cells. J. Agric. Food Chem., 859–864 (2008).
Jaeger, A., Wälti, M., and Neftel, K., Side effects of flavonoids in medical practice. Prog. Clin. Biol. Res., 280, 379–394 (1988).
Kim, J. Y., Jung, K. J., Choi, J. S., and Chung, H. Y., Hesperetin: a potent antioxidant against peroxynitrite. Free Radic. Res., 38, 761–769 (2004).
Marklund, S. and Marklund, G., Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem., 47, 469–474 (1974).
Moreira, P. I., Siedlak, S. L., Aliev, G., Zhu, X., Cash, A. D., Smith, M. A., and Perry, G., Oxidative stress mechanisms and potential therapeutics in Alzheimer disease. J. Neural Transm., 112, 921–932 (2005).
Nunomura, A., Moreira, P. I., Lee, H. G., Zhu, X., Castellani, R. J., Smith, M. A., and Perry, G., Neuronal death and survival under oxidative stress in Alzheimer and Parkinson diseases. CNS Neurol. Disord. Drug Targets, 6, 411–423 (2007).
Ohkawa, H., Ohishi, N., and Yagi, K., Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem., 95, 351–358 (1979).
Pollard, S. E., Whiteman, M., and Spencer, J. P., Modulation of peroxynitrite-induced fibroblast injury by hesperetin: a role for intracellular scavenging and modulation of ERK signaling. Biochem. Biophys. Res. Commun., 347, 916–923 (2006).
Rao, A. V. and Balachandran, B., Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutr. Neurosci., 5, 291–309 (2002).
Reznick, A. Z. and Packer, L., Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol., 233, 357–363 (1994).
Ross, J. A. and Kasum, C. M., Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu. Rev. Nutr., 22, 19–34 (2002).
Sian, J., Dexter, D. T., Lees, A. J., Daniel, S., Agid, Y., Javoy-Agid, F., Jenner, P., and Marsden, C. D., Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol., 36, 348–355 (1994).
Suk, K., Regulation of neuroinflammation by herbal medicine and its implications for neurodegenerative diseases. A focus on traditional medicines and flavonoids. Neurosignals., 14, 23–33 (2005).
Zhu, Y., Carvey, P. M., and Ling, Z., Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res., 1090, 35–44 (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, E.J., Ahn, W.S. Neuroprotective effects of chronic hesperetin administration in mice. Arch. Pharm. Res. 31, 1457–1462 (2008). https://doi.org/10.1007/s12272-001-2130-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-001-2130-1