Abstract
The present study investigated the chemical composition of the essential oil (EO) from aerial parts (flowering stage) of Achillea wilhelmsii C. Koch by GC–MS. In addition, the antioxidant activity of the EO as well as its antimicrobial activity against methicillin-susceptible and methicillin-resistant Staphylococcus aureus (MRSA) strains was tested. Antioxidant activity was measured by the ability of the EO to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals while the antimicrobial activity was assessed by the disc-diffusion method. In total, 52 compounds were recognized, accounting for 97.33 % of the EO. The main compounds in the EO were carvacrol (22.49 %), dihydrocarvone (13.23 %), linalool (12 %), 1,8-cineol (11.42 %), camphene (8.31 %), thymol (5.28 %), camphor (3.71 %), pulegone (2.82 %) α-terpineol (2.11 %), bornyl acetate (1.14 %), and farganol (1.01 %). The EC50 value of the EO was 0.01 and 0.08 mg/mL for the antioxidant and DPPH-scavenging ability, respectively. A. wilhelmsii EO affected methicillin-sensitive Staphylococcus aureus (MSSA) and MRSA, but the impact was more effective on MSSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herbal medicines are considered to be an important natural medicine for the treatment of health conditions and diseases. The excessive and repeated use of the same drugs used in modern medicine has led to the evolution of antibiotic-resistant microbes, including Staphylococcus aureus whose emergence of antibiotic-resistant strains reduces the number of antibiotics available to treat clinical infections caused by this bacterium (Parker and Jevons 1964). S. aureus is a highly versatile pathogen with considerable importance in human medicine. S. aureus is responsible for a wide range of hospital and community-acquired infections worldwide, from skin infections and food poisoning to life-threatening situations such as toxic-shock syndrome, endocarditis, pneumonia, bacteraemia and osteomyelitis (Kim et al. 2006; Akineden et al. 2008). Traditional medicine involving herbs (or the compounds within them) can solve health and medical problems caused by S. aureus. The essential oils (EOs) (primarily from leaves) of Thymus vulgaris and Eucalyptus globulus were tested against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA), both EOs to possessing antibacterial activity against MRSA, the former being more potent than the latter (Tohidpour et al. 2010). Daucus crinitus (a medicinal plant) EOs (derived from stems and leaves of wild plants) inhibited S. aureus (Bendiabdellah et al. 2013).
Achillea wilhelmsii C. Koch a perennial medicinal herb belonging to the Asteraceae family has a relatively wide distribution in different parts of Iran (Rechinger 1963; Mozaffarian 1966). It is native to Western Asia and Europe, although populations have also been discovered in North America, Australia and New Zealand (Dokhani et al. 2005). A. wilhelmsii has a wide range of reported biological activities, including antispasmodic (Yaeesh et al. 2006), antacid (Niazmand et al. 2010), antioxidant (Candan et al. 2003; Baris et al. 2006; Nemeth and Bernath 2008; Fathi et al. 2011), antihyperlipidemia (Asgary et al. 2000), antihypertensive (Niazmand et al. 2011) and antitumoral (Csupor-Loffler et al. 2009).
The hygiene industry utilizes A. wilhelmsii EO to make skin tender and soft and to treat skin inflammations using cream formulations (Pieroni et al. 2004). A. wilhelmsii is rich in sesquiterpenes, lactones flavonoids and monoterpenoids which have antioxidant activities (Jaimand and Rezaee 2001; Saeidinia et al. 2005).
The main purpose of the present study was to perform a biological examination on A. wilhelmsii C. Koch from Golmakan Khorasan Razavi, Iran by assessing the antioxidant and antimicrobial activity of the EO.
Materials and methods
Plant preparation and procedure
Aerial parts (stems, leaves and flowers) of the flowering stage of A. wilhelmsii C. Koch (Fig. 1a, b) were collected in June 2012 from Golmakan (36°28′44″N, 59°9′17″E), Khorasan Razavi, Iran. The plant was taxonomically identified by a botanist at the herbarium of Pharmacognosy, Department of the Faculty of Pharmacy affiliated to Shahid Beheshti University of Medical Sciences of Iran.
Extraction and isolation of the EO
Plant parts were air-dried in the shade at ambient temperature (18–25 °C) for 12 days. Dried aerial parts (100 g) were cut into small pieces and hydro-distilled for 4 h using Clevenger-type apparatus. The resulting EO was dried over anhydrous sodium sulfate and stored at 4 °C until GC–MS analysis and bioassays.
GC–MS analysis
In this study, an HP 6890 N GC system coupled with an HP MSD5973 N quadruple mass spectrometer was utilized. The extracted compounds were separated on an HP-5MS capillary column (30 m length, 0.25 mm internal diameter, 0.25 mm film thickness). Split injection:sample ratio for distillation was 50:1. The column oven temperature was programmed to rise from an initial 40 to 150 °C at 4 °C/min, and then to 240 °C at 10 °C/min. Injection temperature and ion source temperature were 240 °C. Helium was used as the carrier gas with a flow rate of 1.2 mL/min. The ionizing energy was 70 eV. All data were obtained by collecting the full-scan mass spectra within the scan range 50–550 amu. Compounds were identified using the Wiley 7n.L Mass Spectral Library (Wiley, New York, NY, USA). Trace compounds were defined as those detected at <0.04 % of the EO.
Antioxidant activity
Antioxidant activity was measured by the paired diene method (Lingnert et al. 1979). The antioxidant activity measured is the ability of the EO to inhibit the peroxidation of linoleic acid in which the double bond is altered to a paired diene. Each EO sample (0.01–30 mg/mL) in methanol (100 μL) was mixed with 3 mL of 10 mM linoleic acid (Sigma Chemical Co., St. Louis, MO, USA) to form an emulsion in 0.2 M sodium phosphate buffer (pH 6.6) in test tubes and placed in the dark at 37 °C to quicken oxidation. After incubation for 17 h, 7 mL of 70 % methanol in deionized water was added, and the absorbance of the mixture was measured at 234 nm against a blank in a Hitachi U-2001 spectrophotometer (Tokyo, Japan). Antioxidant activity was quantified as follows: Antioxidant activity (%) = [(∆A234 of control − ∆A234 of sample)/∆A234 of control] × 100. Analyses were repeated three times. α-Tocopherol, butylated hydroxyanisole (BHA) and ascorbic acid (Sigma) were used as standard controls.
Scavenging ability on 1, 1-diphenyl-2-picrylhydrazyl radicals
The scavenging ability of 1,1-diphenyl-2-picrylhydrazyl (DPPH, Sigma) radicals, which was measured by the method of Shimada et al. (1992), is the ability of the EO to react quickly with DPPH radicals and to decrease most DPPH radical molecules. The assay was repeated three times. α-Tocopherol, BHA and ascorbic acid were used as standard controls. Each EO sample (0.5–30 mg/mL) in methanol (5 mL) was mixed with 1 mL of methanolic solution containing DPPH radicals, resulting in a final concentration of 0.2 mM DPPH. The mixture was shaken vigorously, left to stand for 45 min in the dark, and the absorbance was then measured at 517 nm against a blank. The scavenging ability was calculated as follows:
Scavenging ability (%) = [(∆A517 of control − ∆A517 of sample)/∆A517 of control] × 100.
EC50 value (mg/mL) is the efficient concentration at which the antioxidant activity was inhibited by 50 % and DPPH radicals were scavenged by 50 %, and was gained by interpolation from linear regression analysis.
Antibacterial study
Preparation of microorganisms
The S. aureus strains utilized in this study were clinical isolates from patients with S. aureus, obtained from the microbiological laboratory of the central hospital in Shiraz, Iran. This study was approved by the ethics committees of Zabol and Shiraz Universities of Medical Sciences. MRSA that were isolated were identified by screening tests on Mueller–Hinton agar (MHA, Torlak, Berlin, Germany) supplemented with 5 % NaCl and 1 mg/mL oxacillin-impregnated disc to isolate MRSA (Roberts et al. 2002). Finally, 10 MRSA strains and 5 methicillin-susceptible S. aureus (MSSA) strains were isolated from patients. In this study, two standards strains, ATTC 25923 (MRSA) and PTCC 1341 (MSSA), were used.
Disc-diffusion assay
Antimicrobial tests were performed by the disc-diffusion method using 100 μL of suspension (containing 2.0 × 108 CFU/mL of bacteria) dispersed evenly on MHA in sterilized Petri dishes (80 mm in diameter). To the discs (6 mm in diameter, HiMedia Laboratories Pvt. Ltd., Mumbai, India), 20, 50, 100 and 200 μL of EO and placed on the inoculated agar. The inoculated plates were maintained at 4 °C for 2 h and incubated at 37 °C for 24 h. Antimicrobial activity was evaluated by measuring the zone of inhibition (mm) against the test bacterial (MRSA and MSSA) strains.
Statistical analysis
The EO was prepared in triplicate for chemical characterization and for antioxidant and antibacterial assays. Data was subjected to analysis of variance following a completely random design to determine the least significant difference (LSD) at P < 0.05 using SPSS v. 11.5.
Results and discussion
The composition of A. wilhelmsii essential oil
The mass spectra and retention indices (RI) were used in this study to determine the EO composition of A. wilhelmsii. In total, 52 compounds were identified accounting for 97.33 % of the EO components (Table 1). The main compounds of the EO were (in decreasing order) carvacrol (22.49 %), dihydrocarvone (13.23 %), linalool (12 %), 1,8-cineol (11.42 %), camphene (8.31 %), thymol (5.28 %), camphor (3.71 %), pulegone (2.82 %), α-pinene (2.2 %), terpineol (2.11 %), bornyl acetate (1.14 %) and farganol (1.01 %). Some compounds were detected in trace amounts (not listed in Table 1): heptanal, isopentyl isovalerate, neo-3-thujanol, cis-jasmone, elemol. The main compound in the EO of A. wilhelmsii from the Golmakan Khorasan Razavi (Iran) area was carvacrol (22.42 %). Javidnia et al. (2004) also found 25.1 % carvacrol in A. wilhelmsii oil as the main compound. The major constituent of the EO of the flowers and leaves of A. wilhelmsii from Mazandaran (Iran) province was camphor, 21.2 % and 24.1 %, respectively (Azadbakht et al. 2003). Carvacrol and camphor have no harmful effects on humans and environment (Rajendran and Sriranjini 2008; Khani and Asgari 2012). The amount of camphor in A. wilhelmsii EO collected from Kerman was 9.0 % (Afsharypuor et al. 1996) and in the EO of aerial parts from Kazeroon (Iran, Fars) province was 2.2 % (Javidnia et al. 2004). Afsharypuor et al. (1996) reported that main compound in the EO of A. wilhelmsii was caryophyllene oxide (12.5 %), much higher than that reported in our study (0.08 %). 1,8-cineol, which was found at 3.32 % of the stem EO in this study was the major constituent of the oil of A. wilhelmsii from Egypt and Turkey (Javidnia et al. 2004; Baris et al. 2006). The variations in the qualitative and quantitative composition of the EOs from different locations within the same country or from different countries are likely caused by genetic variation, growth conditions, geographic variation and analytical protocols used to assess the EOs. Previous studies showed that monoterpenes, the main part of the A. wilhelmsii EO from Golmakan, have influential insecticidal effects against stored product insects (Papachristos et al. 2004; Rajendran and Sriranjini 2008). Consequently, the A. wilhelmsii EO from Golmakan could be an important optional phytochemical control strategy without undesirable effects such as direct toxicity to humans and environmental pollution (Rajendran and Sriranjini 2008; Khani and Asgari 2012). In addition, A. wilhelmsii EO contains sesquiterpenes, lactones and flavonoids, which have in the ability to lower blood lipid levels and hypertension (Asgary et al. 2000).
Antioxidant activity and scavenging ability
The results for antioxidant activity and scavenging ability on DPPH radicals of the EOs assayed are summarized in Table 2. The efficiency of antioxidant activity and scavenging ability is inversely related with their EC50 values. The antioxidant activity EC50 values were 0.08, 0.05, 4.07 and 0.01 mg/mL for α-tocopherol, BHA, ascorbic acid and A. wilhelmsii EO, respectively. The scavenging ability EC50 values was 0.11, 0.07, 9.09 and 0.58 mg/mL for α-tocopherol, BHA, ascorbic acid and A. wilhelmsii EO, respectively. The antioxidant activity of A. wilhelmsii EO was stronger than three standards tested (Table 2), which could be used for the treatment of human diseases to remove free radicals (Dharmendra et al. 2009).
Antibacterial activity
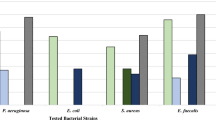
The results of antibacterial activity of EOs (Table 3) showed that the maximum level of EO (200 μL) was inhibitory (largest zone of inhibition) against MSSA (22.56 mm) and MRSA (14.22 mm). The inhibitory activity against MSSA was greater than against MRSA. The EOs of A. wilhelmsii had a more negative impact on MSSA than MRSA (Table 3). Monoterpenes, which are rich in the EO of A. wilhelmsii, have powerful antibacterial effects (Unlu et al. 2002; Sokmen et al. 2004; Prabuseenivasan et al. 2006). In addition, phenolic and flavonoid compounds, also present in the EO of A. wilhelmsii, have antimicrobial activity (Stojanovic et al. 2005; Eleyinmi 2007; Yaghoubi et al. 2007; Mothana et al. 2009).
Conclusion
In this study, chemical composition, antioxidant activity and in vitro antibacterial activity of A. wilhelmsii L. Essential oil on methicillin-susceptible and methicillin-resistant S. aureus spp. was investigated. A. wilhelmsii C. Koch has emerged as an important medicinal plant. Its EO could be commercialized for its antioxidant, insecticidal and antibacterial applications, or used in the pharmaceutical, cosmetic or perfume industries.
Abbreviations
- A. wilhelmsii :
-
Achillea wilhelmsii
- BHA:
-
Butylated hydroxyanisole
- EO:
-
Essential oil
- DPPH 1:
-
1-Diphenyl-2-picrylhydrazyl
- GC–MS:
-
Gas chromatography–mass spectrometry
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- MSSA:
-
Methicillin-sensitive Staphylococcus aureus
- S. aureus :
-
Staphylococcus aureus
References
Afsharypuor S, Asgaryand S, Lockwood GB (1996) Constituents of the essential oil of Achillea wilhelmsii from Iran. Planta Med 62(1):77–78. doi:10.1055/s-2006-957810
Akineden O, Hassan AA, Schneide E, Usleber E (2008) Enterotoxigenic properties of Staphylococcus aureus isolated from goats’ milk cheese. Int J Food Microbiol 124(2):211–216. doi:10.1016/j.ijfoodmicro.2008.03.027
Asgary S, Naderi GH, Sarrafzadegan N, Mohammadifard N, Mostafavi S, Vakili R (2000) Antihypertensive and antihyperlipidemic effects of Achillea wilhelmsii. Drugs Exp Clin Res 26(3):89–93
Azadbakht M, Morteza-Semnani K, Khansari N (2003) The essential oil composition of Achillea wilhelmsii C. Koch. leaves and flowers. J Med Plants 2:55–59
Baris O, Culluce M, Sahin F, Ozer H, Kilic H, Ozkan H, Skmen M, Zbek T (2006) Biological activities of the essential oil and methanol extract of Achillea biebersteinii Afan. (Asteraceae). Turk J Biol 30:65–73
Bendiabdellah A, Amine Dib MEL, Meliani N, Muselli A, Nassim D, Tabti B, Costa J (2013) Antibacterial activity of Daucus crinitus essential oils along the vegetative life of the plant. J Chem 1:7–12. doi:10.1155/2013/149502
Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sokmen A, Akpulat HA (2003) Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J Ethnopharmacol 87(2–3):215–220. doi:10.1016/S0378-8741(03)00149-1
Csupor-Loffler B, Hajdu Z, Zupko I, Rethy B, Falkay G, Forgo P, Hohmann J (2009) Antiproliferative effect of flavonoids and sesquiterpenoids from Achillea millefolium S.L. on cultured human tumour cell lines. Phytother Res 23(5):672–676. doi:10.1002/ptr.2697
Dharmendra D, Prashant K, Jain SK (2009) In-vitro anti-oxidant activity of the ethyl acetate extract of gum guggul (Commiphora Mukul). Biological Forum 1(1):32–35
Dokhani S, Cottrell T, Khajeddin J, Mazza G (2005) Analysis of aroma and phenolic components of selected Achillea species. Plant Food Hum Nutr 6:55–62. doi:10.1007/s11130-005-5100-9
Eleyinmi AF (2007) Chemical composition and antibacterial of Gongronema latifolium. J Zhejiang Univ Sci B 8:352–358. doi:10.1631/jzus.2007.B0352
Fathi H, Lashtoo Aghaee B, Ebrahimzadeh MA (2011) Antioxidant activity and phenolic contents of Achillea wilhelmsii. Pharmacol Online 2:942–949
Jaimand K, Rezaee MB (2001) Comparative study of the essential oils of three Achillea species from Iran. J Essent Oil Res 13:354–356. doi:10.1080/10412905.2001.9712231
Javidnia K, Miri R, Sadeghpour H (2004) Composition of the volatile oil of Achillea wilhelmsii c. Koch from Iran. Daru 12:2–9
Khani A, Asgari J (2012) Insecticide activity of essential oils of Mentha longifolia, Pulicaria gnaphalodes and Achillea wilhelmsii against two stored product pests, the flour beetle, Tribolium castaneum and the cowpea weevil, Callosobruchus maculatus. J Insect Sci 12:102–107. doi:10.1673/031.012.7301
Kim JS, Song W, Kim HS, Cho HC, Lee KM, Choi MS, Kim EC (2006) Association between the methicillin resistance of clinical isolates of Staphylococcus aureus, their staphylococcal cassette chromosome mec (SCCmec) subtype classification and their toxin gene profiles. J Microbiol Infect Dis 56:289–295. doi:10.1016/j.diagmicrobio.2006.05.003
Lingnert H, Vallentin K, Eriksson CE (1979) Measurement of antioxidative effect in model system. J Food Process Preserv 3:87–103. doi:10.1111/j.1745-4549.1979.tb00574.x
Mothana R, Lindequist U, Geraenert R, Bednarski P (2009) Studies of the in vitro anticancer, antimicrobial and antioxidant potentials of selected Yemeni medicinal plants from the island Soqotra. BMC Complement Altern Med 9:7–11. doi:10.1186/1472-6882-9-7
Mozaffarian V (1966) A dictionary of Iranian plant names: Latin–English–Persian. Farhang Mo’aser
Nemeth E, Bernath J (2008) Biological activities of yarrow species (Achillea spp.). Curr Pharm Des 14(29):3151–3167. doi:10.2174/138161208786404281
Niazmand S, Khooshnood E, Derakhshan M (2010) Effects of Achillea wilhelmsii on rat’s gastric acid output at basal, vagotomized, and vagal-stimulated conditions. Pharmacogn Mag 6(24):282–285. doi:10.4103/0973-1296.71791
Niazmand S, Esparham M, Rezaee SA, Harandizadeh F (2011) Hypotensive effect of Achillea wilhelmsii aqueous-ethanolic extract in rabbit. Aveicennal J Phytomed 1:51–56
Papachristos DP, Karamanoli KI, Stamopoulos DC, Menkissoglu-Spiroudi U (2004) The relationship between the chemical composition of three essential oils and their insecticidal activity against Acanthoscelides obtectus (Say). Pest Manag Sci 60(5):514–520. doi:10.1002/ps.798
Parker MT, Jevons MP (1964) A survey of methicillin resistance in Staphylococcus aureus. Postgrad Med J 40:170–178. doi:10.1136/pgmj.40.Suppl.170
Pieroni A, Quave CL, Villanelli ML, Mangino P, Sabbatini G, Santini L, Boccetti T, Profili M, Ciccioli T, Rampa LG, Antonini G, Girolamini C, Cecchi M, Tomasi M (2004) Ethnopharmacognostic survey on the natural ingredients used in folk cosmetics, cosmeceuticals and remedies for healing skin diseases in the inland Marches. Central-Eastern Italy. J Ethnopharmacol 91(2–3):331–344. doi:10.1016/j.jep.2004.01.015
Prabuseenivasan S, Jayakumar M, Ignacimuthu S (2006) In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med 6:39. doi:10.1186/1472-6882-6-39
Rajendran S, Sriranjini V (2008) Plant products as fumigant for stored product insect control. J Stored Prod Res 44(2):126–135. doi:10.1016/j.jspr.2007.08.003
Rechinger KH (1963) Flora Iranica, Wien, Austria. Akademische Druke- U. Verlagsanstatt.
Roberts S, Young H, Faulkner S, Renshaw S, Morris AJ (2002) Value of broth cultures in detecting methicillin-resistant Staphylococcus aureus. NZ Med J 115:1–2
Saeidinia S, Gohari AR, Yassa N, Shafiee A (2005) Composition of the volatile oil of Achillea conferta DC. from Iran. Daru 13:34–36
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948. doi:10.1021/jf00018a005
Sokmen A, Sokmen M, Daferera D, Polissiou M, Candan F, Unlu M, Akpulat HA (2004) The in vitro antioxidant and antimicrobial activities of the essential oil and methanol extracts of Achillea biebersteini Afan. (Asteraceae). Phytother Res 18:451–456. doi:10.1002/ptr.1438
Stojanovic G, Radulovic N, Hashimoo T, Palic R (2005) In vitro antimicrobial activity of extracts of four Achillea species: the composition of Achillea clavennae L. (Asteraceae) extract. J Ethnopharmacol 101:185–190. doi:10.1016/j.jep.2005.04.026
Tohidpour A, Sattari M, Omidbaigi R, Yadegar A, Nazemi J (2010) Antibacterial effect of essential oils from two medicinal plants against methicillin-resistant Staphylococcus aureus (MRSA). Phytomedicine 17(2):142–145. doi:10.1016/j.phymed.2009.05.007
Unlu M, Daferera D, Donmez E, Polissiou M, Tepe B, Sokmen A (2002) Compositions and the in vitro antimicrobial activities of the essential oils of Achillea setacea and Achillea teretifolia (Compositae). J Ethnopharmacol 83:117–121. doi:10.1016/S03788741(02)00218-0
Yaeesh S, Jamal Q, Khan AU, Gilani AH (2006) Studies on hepatoprotective, antispasmodic and calcium antagonist activities of the aqueous-methanol extract of Achillea millefolium. Phytother Res 20(7):546–551. doi:10.1002/ptr.1897
Yaghoubi SMJ, Ghorbani GR, Soleimanianzad S, Satari R (2007) Antimicrobial activity of Iranian propolis and its chemical composition. Daru 15:24–48
Acknowledgments
The authors are grateful to Professor Hildebert Wagner Center of Pharma-Research, Institute of Pharmacy LM-University of Munich, Germany, for his valuable comments on the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Alfatemi, S.M.H., Rad, J.S., Rad, M.S. et al. Chemical composition, antioxidant activity and in vitro antibacterial activity of Achillea wilhelmsii C. Koch essential oil on methicillin-susceptible and methicillin-resistant Staphylococcus aureus spp.. 3 Biotech 5, 39–44 (2015). https://doi.org/10.1007/s13205-014-0197-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13205-014-0197-x