Abstract
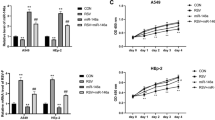
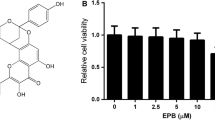
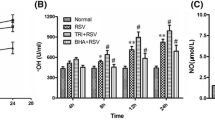
The present study investigated the role of colchicine in the treatment of RSV infection. Treatment of BEAS-2B cells following RSV infection with colchicine caused a significant decrease in the number of viral plaques. In RSV-infected BEAS-2B cells’ treatment with colchicine leads to a significant up-regulation of both IFN-β1 and RIG-I genes. The levels of interleukin, NO, and MDA were suppressed in BEAS-2B cells infected with RSV by colchicine. The phosphorylation of Stat3, COX-2, and p38 was also suppressed significantly by colchicine. The phosphorylation of IkBα was promoted in RSV-infected BEAS-2B cells’ oncolchicine treatment. In neonatal rats, replication of RSV was inhibited significantly by colchicine treatment which was evident by suppression of RSV-L gene expression. A significant decrease in the level of IL-6 and TNF-α was caused in neonatal rat BALF by colchicine treatment. The production of MDA, NO and MPO in the neonatal rat BALF was suppressed markedly by colchicine treatment. Treatment of the neonatal rats infected by RSV with colchicine suppressed the release of IκBα and COX-2 in the pulmonary epithelial cells. Colchicine treatment of the neonatal rats promoted the expression of IFN-α and IFN-β1. In summary, the current study showed that colchicine inhibited RSV infection in neonatal rats through regulation of anti-oxidative factor production. The expression of IFN-β1 and RIG-I genes was also up-regulated in the RSV-infected alveolar epithelial cells by treatment with colchicine. Therefore, colchicine may be developed as the therapeutic agent for the treatment of RSV infection.







Similar content being viewed by others
References
Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM (2002) Critical role for CXCR1 and CXCR1 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest 110:1703–1716
Collins PL, Graham BS (2008) Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol 82:2040–2055
Disel U, Paydas S, Dogan A, Gulfiliz G, Yavuz S (2004) Effect of colchicine on cyclosporine nephrotoxicity, reduction of TGF–beta overexpression, apoptosis, and oxidative damage: an experimental animal study. Transpl Proc 36:1372–1376
Feng Q, Su Z, Song S, Χu H, Zhang B, Yi L, Tian M, Wang H (2016) Histone deacetylase inhibitors suppress RSV infection and alleviate virus-induced airway inflammation. Int J Mol Med 38:812–822
Guan T, Gao B, Chen G, Chen X, Janssen M, Uttarwar L, Ingram AJ, Krepinsky JC (2013) Colchicine attenuates renal injury in a model of hypertensive chronic kidney disease. Am J Physiol Renal Physiol 305:F1466–F1476
Hall CB, Geiman JM, Biggar R, Kotok DI, Hogan PM, Douglas GR Jr (1976) Respiratory syncytial virus infections within families. N Engl J Med 294:414–419
Hastie SB (1991) Interactions of colchicine with tubulin. Pharmacol Ther 51:377–401
Hosakote YM, Komaravelli N, Mautemps N, Liu T, Garofalo RP, Casola A (2012) Antioxidant mimetics modulate oxidative stress and cellular signaling in airway epithelial cells infected with respiratory syncytial virus. Am J Physiol Lung Cell Mol Physiol 303:L991–L1000
Icardi L, Lievens S, Mori R, Piessevaux J, De Cauwer L, De Bosscher K, Tavernier J (2012) Opposed regulation of type I IFN-induced STAT3 and ISGF3 transcriptional activities by histone deacetylases (HDACS) 1 and 2. FASEB J 26:240–249
Jamaluddin M, Tian B, Boldogh I, Garofalo RP, Brasier AR (2009) Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. J Virol 83:10605–10615
Jie YH, Cammisuli S, Baggiolini M (1984) Immunomodulatory effects of Panax Ginseng C.A. Meyer in the mouse. Agents Actions 15:386–391
Ledwozyw A (1994) The effect of colchicine and vinblastine on bleomycin–induced lung fibrosis in rats. Acta Physiol Hung 82:383–389
Li JJ, Lee SH, Kim DK, Jin R, Jung DS, Kwak SJ, Kim SH, Han SH, Lee JE, Moon SJ et al (2009) Colchicine attenuates inflammatory cell infiltration and extracellular matrix accumulation in diabetic nephropathy. Am J Physiol Renal Physiol 297:F200–F209
McClurkin C Jr, Phan SH, Hsu CH, Patel SR, Spicker JK, Kshirsagar AM, Yuan WY, Wiggins RC (1990) Moderate protection of renal function and reduction of fibrosis by colchicine in a model of anti–GBM disease in the rabbit. J Am Soc Nephrol 1:257–265
Monick M, Staber J, Thomas K, Hunninghake G (2001) Respiratory syncytial virus infection results in activation of multiple protein kinase C isoforms leading to activation of mitogen-activated protein kinase. J Immunol 166:2681–2687
Nuki G (2008) Colchicine: its mechanism of action and efficacy in crystal–induced inflammation. Curr Rheumatol Rep 10:218–227
Rodríguez L, Cerbón-Ambriz J, Muñoz ML (1998) Effects of colchicine and colchiceine in a biochemical model of liver injury and fibrosis. Arch Med Res 29:109–116
Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW (2005) Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol 79:3350–3357
Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ (1999) Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 282:1440–1446
Slobodnick A, Shah B, Pillinger MH, Krasnokutsky S (2015) Colchicine: old and new. Am J Med 128:461–470
Wang H, Peters N, Schwarze J (2006) Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J Immunol 177:6263–6270
Wang YCYX, Yang X, Xing LH, Kong WZ (2013) Effects of SAHA on proliferation and apoptosis of hepatocellular carcinoma cells and hepatitis B virus replication. World J Gastroenterol 19:5159–5164
Zeng R, Li C, Li N, Wei L, Cui Y (2011) The role of cytokines and chemokines in severe respiratory syncytial virus infection and subsequent asthma. Cytokine 53:1–7
Acknowledgements
This study was supported by Projects in Yunnan Science and Technology Plan (No: 2017FB147).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, N., Yang, Y., Liu, H. et al. Inhibition of respiratory syncytial virus replication and suppression of RSV-induced airway inflammation in neonatal rats by colchicine. 3 Biotech 9, 392 (2019). https://doi.org/10.1007/s13205-019-1917-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-019-1917-z