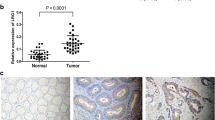
Abstract
Aberrant expression of Liver X receptor α (LXRα) has been frequently reported in various types of cancers excluding gastric cancer (GC). Moreover, the role of LXRα in human GC has not been previously reported. In this study, we investigated the effect of LXRα down-regulation on invasion and EMT of GC. The expression of LXRα in GC cell lines was detected by real-time PCR. The LXRα siRNA was transiently transfected into GC cells using Lipofectamine™ 2000 reagent. Subsequently, cell invasive ability was evaluated by Transwell assays. Western blot and real-time PCR were used to determined the expressions of matrix metalloproteinase-2 and -9 (MMP-2 and -9), E-cadherin, N-cadherin, Vimentin, Snail, Slug, and Twist in GC cells. In addition, the effect of LXRα down-regulation on the phosphoinositide 3-kinase (PI3K)/Akt/nuclear factor (NF)-κB signaling pathway was explored by Western blot. From our results, we found that the expression of LXRα was significantly increased in GC tissues and cell lines. Knockdown of LXRα suppressed the invasive ability of GC cells. The levels of MMP-2 and -9 were dramatically decreased by down-regulating LXRα. In addition, we found a decrease of N-cadherin, Twist, and Slug expressions and an increase of E-cadherin expression, but no influence on the expression levels of Vimentin and Snail. We also found that LXRα down-regulation might suppress the phosphorylation of Akt, NF-κB, and IκB. Collectively, our results indicated that down-regulation of LXRα was shown to suppress invasion and EMT of GC cells by decreasing the expressions of related proteins through inhibiting the PI3K/Akt/NF-κB signaling pathway.





Similar content being viewed by others
References
Lee SH, Kim IH, Kim IH, et al. Comparison of short-term outcomes and acute inflammatory response between laparoscopy-assisted and totally laparoscopic distal gastrectomy for early gastric cancer. Ann Surg Treat Res. 2015;89(4):176–82.
Choi AH, Nelson RA, Merchant SJ, et al. Rates of lymph node metastasis and survival in T1a gastric adenocarcinoma in Western populations. Gastrointest Endosc. 2015;83(6):1184–92.
Subhash VV, Yeo MS, Tan WL, et al. Strategies and advancements in harnessing the immune system for gastric cancer immunotherapy. J Immunol Res. 2015;. doi:10.1155/2015/308574.
Price JT, Thompson EW. Mechanisms of tumour invasion and metastasis: emerging targets for therapy. Expert Opin Ther Targets. 2002;6(2):217–33.
Simpson-Haidaris PJ, Rybarczyk B. Tumors and fibrinogen. The role of fibrinogen as an extracellular matrix protein. Ann N Y Acad Sci. 2001;936:406–25.
Klein G, Vellenga E, Fraaije MW, et al. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, eg acute leukemia. Crit Rev Oncol Hematol. 2004;50(2):87–100.
Zucker S, Vacirca J, et al. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23(1–2):101–17.
Yang J, Weinberg RA, et al. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–29.
Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8.
Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23(10):912–23.
Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–37.
Sánchez-Tilló E, Liu Y, de Barrios O, et al. EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci. 2012;69(20):3429–56.
Bhui K, Tyagi S, Srivastava AK, et al. Bromelain inhibits nuclear factor κ-B translocation, driving human epidermoid carcinoma A431 and melanoma A375 cells through G(2)/M arrest to apoptosis. Mol Carcinog. 2012;51(3):231–43.
Neumann M, Naumann M. Beyond IκBs: alternative regulation of NF-κB activity. FASEB J. 2007;21(11):2642–54.
Wang Y, Zhou Y, Jia G, et al. Shikonin suppresses tumor growth and synergizes with gemcitabine in a pancreatic cancer xenograft model: involvement of NF-κB signaling pathway. Biochem Pharmacol. 2014;88(3):322–33.
Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25(51):6680–4.
Wei PL, Tu CC, Chen CH, et al. Shikonin suppresses the migratory ability of hepatocellular carcinoma cells. J Agric Food Chem. 2013;61(34):8191–7.
Song FN, Duan M, Liu LZ, et al. RANKL promotes migration and invasion of hepatocellular carcinoma cells via NF-κB-mediated epithelial-mesenchymal transition. PLoS One. 2014;9(9):e108507.
Willy PJ, Umesono K, Ong ES, et al. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9(9):1033–45.
Teboul M, Enmark E, Li Q, et al. OR-1, a member of the nuclear receptor superfamily that interacts with the 9-cis-retinoic acid receptor. Proc Natl Acad Sci. 1995;92(6):2096–100.
Repa JJ, Turley SD, Lobaccaro JA, et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289(5484):1524–9.
Zelcer N, Hong C, Boyadjian R, et al. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325(5936):100–4.
Zelcer N, Tontonoz P, et al. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116(3):607–14.
Bovenga F, Sabbà C, Moschetta A, et al. Uncoupling nuclear receptor LXR and cholesterol metabolism in cancer. Cell Metab. 2015;21(4):517–26.
Lin CY, Gustafsson JÅ. Targeting liver X receptors in cancer therapeutics. Nat Rev Cancer. 2015;15(4):216–24.
Lo Sasso G, Bovenga F, Murzilli S, et al. Liver X receptors inhibit proliferation of human colorectal cancer cells and growth of intestinal tumors in mice. Gastroenterology. 2013;144(7):1497–507.
Vedin LL, Gustafsson JÅ, Steffensen KR. The oxysterol receptors LXRα and LXRβ suppress proliferation in the colon. Mol Carcinog. 2013;52(11):835–44.
Vedin LL, Lewandowski SA, Parini P, et al. The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis. 2009;30(4):575–9.
Kaneko T, Kanno C, Ichikawa-Tomikawa N, et al. Liver X receptor reduces proliferation of human oral cancer cells by promoting cholesterol efflux via up-regulation of ABCA1 expression. Oncotarget. 2015;6(32):33345–57.
Chang YW, Zhao YF, Cao YL, et al. Liver X receptor α inhibits osteosarcoma cell proliferation through up-regulation of FoxO1. Cell Physiol Biochem. 2013;32(1):180–6.
Hu C, Liu D, Zhang Y, et al. Specific LXRs agonists downregulated expression of FOXM1, cyclin D1 and cyclin B1 in hepatocellular carcinoma (HCC) cells, which led to cell cycle and cell proliferation arrest. Oncogene. 2014;33(22):2888–97.
Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42.
Woessner Jr JF. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5(8):2145–54.
Basset P, Okada A, Chenard MP, et al. Matrix metalloproteinases as stromal effectors of human carcinoma progression: therapeutic implications. Matrix Biol. 1997;15(8–9):535–41.
Nelson AR, Fingleton B, Rothenberg ML, et al. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18(5):1135–49.
Chung TW, Moon SK, Lee YC, et al. Enhanced expression of matrix metalloproteinase-9 by hepatitis B virus infection in liver cells. Arch Biochem Biophys. 2002;408(2):147–54.
Chung TW, Lee YC, Ko JH, et al. Hepatitis B Virus X protein modulates the expression of PTEN by inhibiting the function of p53, a transcriptional activator in liver cells. Cancer Res. 2003;63(13):3453–8.
Kohn EC, Liotta LA. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995;55(9):1856–62.
Graham TR, Odero-Marah VA, Chung LW, et al. PI3K/Akt-dependent transcriptional regulation and activation of BMP-2-Smad signaling by NF-κB in metastatic prostate cancer cells. Prostate. 2009;69(2):168–80.
Yu J, Wang Q, Wang H, et al. Activation of liver X receptor enhances the proliferation and migration of endothelial progenitor cells and promotes vascular repair through PI3k/Akt/eNOS signaling pathway activation. Vasc Pharmacol. 2014;62(3):150–61.
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ji, L., Zhang, B. & Zhao, G. Liver X receptor α (LXRα) promoted invasion and EMT of gastric cancer cells by regulation of NF-κB activity. Human Cell 30, 124–132 (2017). https://doi.org/10.1007/s13577-016-0157-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-016-0157-3