Abstract
Purpose
This review aims to focus an update of the state-of-the-art theranostics approach in liver cancer using 131I, 32P, 90Y, 166Ho, and 186/188Re microspheres as well as some receptors including fibroblast activation protein (FAP), prostate-specific membrane antigen (PSMA), chemokine C-X-C motif receptor 4 (CXCR4), and somatostatin receptors (STR).
Methods
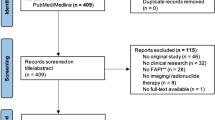
A literature search was performed using the PubMed/MEDLINE and Clinical Trials.gov database and “liver”, “theranostics”, “transarterial radioembolization (TARE)”, “prostate-specific membrane antigen (PSMA)”, “fibroblast activation protein (FAP)”, “hepatocellular carcinoma”, “32P”, “90Y”, “166Ho”, “131I” and “somatostatin receptor (STR)” as keywords. Both, preclinical and clinical studies were included in this review. The search was restricted to the English language.
Result
After exclusion of letters, editorials, comments and duplicates publications, remaining articles were included in this review.
Conclusion
There is growing evidence for applicability of theranostic approach in these patients to enhance early identification, patient evaluation, targeted treatment, restaging, follow-up, and palliation of symptoms or slow down tumor progression. However, this field requires multidisciplinary expertise and collaboration. The increased access to tracers, training multidisciplinary teams, using novel trial designs, and establishing initiatives to share and re-analyze data from clinical imaging studies can encourage the use of molecular nuclear imaging in both clinical research and practical. It is also well recognized that dosimetric recommendations should be considered to compare promising new theranostic agents and clarify the better or similar performance of these methods in hepatocellular carcinoma.










Similar content being viewed by others
References:
https://gco.iarc.fr/today/home. [Online]. Available: https://gco.iarc.fr/today/home.
Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J (2018) Guidelines for diagnosis and treatment of primary liver cancer in China. Liver Cancer 7(3):235–260. https://doi.org/10.1159/000488035
Salem R, Thurston KG (2006) Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J Vasc Interv Radiol 17(8):1251–1278. https://doi.org/10.1097/01.RVI.0000233785.75257.9A. (Erratum. In: J Vasc Interv Radiol 2006 Oct; 17(10):1594 PMID: 16923973)
Salem R, Parikh P, Atassi B, Lewandowski RJ, Ryu RK, Sato KT, Gates VL, Ibrahim S, Mulcahy MF, Kulik L, Liu DM, Riaz A, Omary RA, Kennedy AS (2008) Incidence of radiation pneumonitis after hepatic intra-arterial radiotherapy with yttrium-90 microspheres assuming uniform lung distribution. Am J Clin Oncol 31(5):431–438. https://doi.org/10.1097/COC.0b013e318168ef65
https://www.cancer.org/research/. [Online]. Available: https://www.cancer.org/research/.
Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J (2022) BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 76(3):681–693. https://doi.org/10.1016/j.jhep.2021.11.018
Mulcahy MF, Lewandowski RJ, Ibrahim SM, Sato KT, Ryu RK, Atassi B, Newman S, Talamonti M, Omary RA, Benson A 3rd, Salem R (2009) Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer 115(9):1849–1858. https://doi.org/10.1002/cncr.24224
Giammarile F, Bodei L, Chiesa C, Flux G, Forrer F, Kraeber-Bodere F, Brans B, Lambert B, Konijnenberg M, Borson-Chazot F, Tennvall J, Luster M (2011) Therapy, Oncology and Dosimetry Committees. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging 38(7):1393–1406. https://doi.org/10.1007/s00259-011-1812-2
Kallini JR, Gabr A, Salem R, Lewandowski RJ (2016) Transarterial radioembolization with Yttrium-90 for the treatment of hepatocellular carcinoma. Adv Ther 33(5):699–714. https://doi.org/10.1007/s12325-016-0324-7
Kim HC (2017) Radioembolization for the treatment of hepatocellular carcinoma. Clin Mol Hepatol 23(2):109–114. https://doi.org/10.3350/cmh.2017.0004
Spyridonidis T, Papathanasiou N, Spyridonidis J, Ntzoumani C, Spyropoulou D, Katsanos K, Apostolopoulos DJ (2020) 90Y-microsphere radioembolization: the method, clinical evidence and perspective. Hell J Nucl Med 23(3):330–338. https://doi.org/10.1967/s002449912210
Li R, Li D, Jia G, Li X, Sun G, Zuo C (2021) Diagnostic performance of theranostic radionuclides used in transarterial radioembolization for liver cancer. Front Oncol 25(10):551622. https://doi.org/10.3389/fonc.2020.551622
Wang H, Zhu W, Ren S, Kong Y, Huang Q, Zhao J, Guan Y, Jia H, Chen J, Lu L, Xie F, Qin L (2021) 68Ga-FAPI-04 Versus 18F-FDG PET/CT in the detection of hepatocellular carcinoma. Front Oncol 25(11):693640. https://doi.org/10.3389/fonc.2021.693640
Ho CL, Chen S, Yeung DW, Cheng TK (2007) Dual-tracer PET/CT imaging in evaluation of metastatic hepatocellular carcinoma. J Nucl Med 48(6):902–909. https://doi.org/10.2967/jnumed.106.036673
Castilla-Lièvre MA, Franco D, Gervais P, Kuhnast B, Agostini H, Marthey L, Désarnaud S, Helal BO (2016) Diagnostic value of combining 11C-choline and 18F-FDG PET/CT in hepatocellular carcinoma. Eur J Nucl Med Mol Imaging 43(5):852–859. https://doi.org/10.1007/s00259-015-3241-0
Kesler M, Levine C, Hershkovitz D, Mishani E, Menachem Y, Lerman H, Zohar Y, Shibolet O, Even-Sapir E (2019) 68Ga-PSMA is a novel PET-CT tracer for imaging of hepatocellular carcinoma: a prospective pilot study. J Nucl Med 60(2):185–191. https://doi.org/10.2967/jnumed.118.214833
Lopci E, Torzilli G, Poretti D, de Neto LJ, Donadon M, Rimassa L, Lanza E, Sabongi JG, Ceriani R, Personeni N, Palmisano A, Pedicini V, Comito T, Scorsetti M, Chiti A (2015) Diagnostic accuracy of 11C-choline PET/CT in comparison with CT and/or MRI in patients with hepatocellular carcinoma. Eur J Nucl Med Mol Imaging 42(9):1399–1407. https://doi.org/10.1007/s00259-015-3079-5
Boucher E, Corbinais S, Rolland Y, Bourguet P, Guyader D, Boudjema K, Meunier B, Raoul JL (2003) Adjuvant intra-arterial injection of iodine-131-labeled lipiodol after resection of hepatocellular carcinoma. Hepatology 38(5):1237–1241. https://doi.org/10.1053/jhep.2003.50473
Boucher E, Bouguen G, Garin E, Guillygomarch A, Boudjema K, Raoul JL (2008) Adjuvant intraarterial injection of 131I-labeled lipiodol after resection of hepatocellular carcinoma: progress report of a case-control study with a 5-year minimal follow-up. J Nucl Med 49(3):362–366. https://doi.org/10.2967/jnumed.107.044750
Lau WY, Lai EC, Leung TW, Yu SC (2008) Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: a prospective randomized trial-update on 5-year and 10-year survival. Ann Surg 247(1):43–48. https://doi.org/10.1097/SLA.0b013e3181571047
Lintia-Gaultier A, Perret C, Ansquer C, Eugène T, Kraeber-Bodéré F, Frampas E (2013) Intra-arterial injection of 131I-labeled lipiodol for advanced hepatocellular carcinoma: a 7 years’ experience. Nucl Med Commun 34(7):674–681. https://doi.org/10.1097/MNM.0b013e32836141a0
Dumortier J, Decullier E, Hilleret MN, Bin-Dorel S, Valette PJ, Boillot O, Partensky C, Letoublon C, Ducerf C, Leroy V, Vuillez JP, Borson-Chazot F (2014) Adjuvant intraarterial lipiodol or 131I-lipiodol after curative treatment of hepatocellular carcinoma: a prospective randomized trial. J Nucl Med 55(6):877–883. https://doi.org/10.2967/jnumed.113.131367
Kossert K, Schrader H (2004) Activity standardization by liquid scintillation counting and half-life measurements of 90Y. Appl Radiat Isot 60(5):741–749. https://doi.org/10.1016/j.apradiso.2003.12.009
Lau WY, Leung WT, Ho S, Leung NW, Chan M, Lin J, Metreweli C, Johnson P, Li AK (1994) Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br J Cancer 70(5):994–999. https://doi.org/10.1038/bjc.1994.436
Grady ED (1979) Internal radiation therapy of hepatic cancer. Dis Colon Rectum 22(6):371–375. https://doi.org/10.1007/BF02586901
Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, de Baere T, Assenat E, Tacher V, Robert C, Terroir-Cassou-Mounat M, Mariano-Goulart D, Amaddeo G, Palard X, Hollebecque A, Kafrouni M, Regnault H, Boudjema K, Grimaldi S, Fourcade M, Kobeiter H, Vibert E, Le Sourd S, Piron L, Sommacale D, Laffont S, Campillo-Gimenez B, Rolland Y (2021) DOSISPHERE-01 Study Group Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol 6(1):17–29. https://doi.org/10.1016/S2468-1253(20)30290-9
Arnold CA, Pezhouh MK, Lam-Himlin D, Pittman ME, VandenBussche C, Voltaggio L (2019) 90Y-TheraSpheres: the new look of Yttrium-90. Am J Surg Pathol 43(5):688–694. https://doi.org/10.1097/PAS.0000000000001223
Camacho JC, Kokabi N, Xing M, Schuster DM, Kim HS (2014) PET response criteria for solid tumors predict survival at three months after intra-arterial resin-based 90Yttrium radioembolization therapy for unresectable intrahepatic cholangiocarcinoma. Clin Nucl Med 39(11):944–950. https://doi.org/10.1097/RLU.0000000000000557
Braat AJ, Smits ML, Braat MN, van den Hoven AF, Prince JF, de Jong HW, van den Bosch MA, Lam MG (2015) 90Y hepatic radioembolization: an update on current practice and recent developments. J Nucl Med 56(7):1079–1087. https://doi.org/10.2967/jnumed.115.157446
Gulec SA, Mesoloras G, Stabin M (2006) Dosimetric techniques in 90Y-microsphere therapy of liver cancer: the MIRD equations for dose calculations. J Nucl Med 47(7):1209–1211
Ho S, Lau WY, Leung TW, Chan M, Ngar YK, Johnson PJ, Li AK (1996) Partition model for estimating radiation doses from yttrium-90 microspheres in treating hepatic tumours. Eur J Nucl Med 23(8):947–952. https://doi.org/10.1007/BF01084369
Garin E, Rolland Y, Edeline J, Icard N, Lenoir L, Laffont S, Mesbah H, Breton M, Sulpice L, Boudjema K, Rohou T, Raoul JL, Clement B, Boucher E (2015) Personalized dosimetry with intensification using 90Y-loaded glass microsphere radioembolization induces prolonged overall survival in hepatocellular carcinoma patients with portal vein thrombosis. J Nucl Med 56(3):339–346. https://doi.org/10.2967/jnumed.114.145177
Lam M, Garin E, Maccauro M, Kappadath SC, Sze DY, Turkmen C, Cantasdemir M, Haste P, Herrmann K, Alsuhaibani HS, Dreher M, Fowers KD, Salem R (2022) A global evaluation of advanced dosimetry in transarterial radioembolization of hepatocellular carcinoma with Yttrium-90: the TARGET study. Eur J Nucl Med Mol Imaging 49(10):3340–3352. https://doi.org/10.1007/s00259-022-05774-0
Tafti BA, Padia SA (2019) Dosimetry of Y-90 microspheres utilizing Tc-99m SPECT and Y-90 PET. Semin Nucl Med 49(3):211–217. https://doi.org/10.1053/j.semnuclmed.2019.01.005
Chiesa C, Mira M, Maccauro M, Spreafico C, Romito R, Morosi C, Camerini T, Carrara M, Pellizzari S, Negri A, Aliberti G, Sposito C, Bhoori S, Facciorusso A, Civelli E, Lanocita R, Padovano B, Migliorisi M, De Nile MC, Seregni E, Marchianò A, Crippa F, Mazzaferro V (2015) Radioembolization of hepatocarcinoma with (90)Y glass microspheres: development of an individualized treatment planning strategy based on dosimetry and radiobiology. Eur J Nucl Med Mol Imaging 42(11):1718–1738. https://doi.org/10.1007/s00259-015-3068-8
Haste P, Tann M, Persohn S, LaRoche T, Aaron V, Mauxion T, Chauhan N, Dreher MR, Johnson MS (2017) Correlation of technetium-99m macroaggregated albumin and Yttrium-90 glass microsphere biodistribution in hepatocellular carcinoma: a retrospective review of pretreatment single photon emission CT and posttreatment positron emission tomography/CT. J Vasc Interv Radiol 28(5):722-730.e1. https://doi.org/10.1016/j.jvir.2016.12.1221
Simon N, Feitelberg S (1967) Scanning bremsstrahlung of yttrium-90 microspheres injected intra-arterially. Radiology 88(4):719–724. https://doi.org/10.1148/88.4.719
Pasciak AS, Bourgeois AC, McKinney JM, Chang TT, Osborne DR, Acuff SN, Bradley YC (2014) Radioembolization and the dynamic role of (90)Y PET/CT. Front Oncol 27(4):38. https://doi.org/10.3389/fonc.2014.00038
Ahmadzadehfar H, Muckle M, Sabet A, Wilhelm K, Kuhl C, Biermann K, Haslerud T, Biersack HJ, Ezziddin S (2012) The significance of bremsstrahlung SPECT/CT after yttrium-90 radioembolization treatment in the prediction of extrahepatic side effects. Eur J Nucl Med Mol Imaging 39(2):309–315. https://doi.org/10.1007/s00259-011-1940-8
Rong X, Du Y, Ljungberg M, Rault E, Vandenberghe S, Frey EC (2012) Development and evaluation of an improved quantitative (90)Y bremsstrahlung SPECT method. Med Phys 39(5):2346–2358. https://doi.org/10.1118/1.3700174.
Tehranipour N, Al-Nahhas A, Canelo R, Stamp G, Woo K, Tait P, Gishen P (2007) Concordant F-18 FDG PET and Y-90 Bremsstrahlung scans depict selective delivery of Y-90-microspheres to liver tumors: confirmation with histopathology. Clin Nucl Med 32(5):371–374. https://doi.org/10.1097/01.rlu.0000259568.54976.bd
Villalobos A, Cheng B, Wagstaff W, Sethi I, Bercu Z, Schuster DM, Brandon DC, Galt J, Kokabi N (2021) Tumor-to-normal ratio relationship between planning technetium-99 macroaggregated albumin and posttherapy Yttrium-90 bremsstrahlung SPECT/CT. J Vasc Interv Radiol 32(5):752–760. https://doi.org/10.1016/j.jvir.2020.12.023
Nickles RJ, Roberts AD, Nye JA, Converse AK, Barnhart TE, Avila-Rodriguez MA, Sundaresan R, Dick DW, Hammas RJ, Thomadsen BR (2004) Assaying and PET imaging of ytrrium-90: 1≫34ppm>0. In: Nuclear Science Symposium Conference Record, 2004 IEEE, Vol. 6, (pp. 3412–3414). Piscataway, NJ: IEEE.
Goedicke A, Berker Y, Verburg FA, Behrendt FF, Winz O, Mottaghy FM (2013) Study-parameter impact in quantitative 90-Yttrium PET imaging for radioembolization treatment monitoring and dosimetry. IEEE Trans Med Imaging 32(3):485–492. https://doi.org/10.1109/TMI.2012.2221135
Werner MK, Brechtel K, Beyer T, Dittmann H, Pfannenberg C, Kupferschläger J (2010) PET/CT for the assessment and quantification of (90)Y biodistribution after selective internal radiotherapy (SIRT) of liver metastases. Eur J Nucl Med Mol Imaging 37(2):407–408. https://doi.org/10.1007/s00259-009-1317-4
Duan H, Khalaf MH, Ferri V, Baratto L, Srinivas SM, Sze DY, Iagaru A (2021) High quality imaging and dosimetry for yttrium-90 (90Y) liver radioembolization using a SiPM-based PET/CT scanner. Eur J Nucl Med Mol Imaging 48(8):2426–2436. https://doi.org/10.1007/s00259-021-05188-4
Selwyn RG, Nickles RJ, Thomadsen BR, DeWerd LA, Micka JA (2007) A new internal pair production branching ratio of 90Y: the development of a non-destructive assay for 90Y and 90Sr. Appl Radiat Isot 65(3):318–327. https://doi.org/10.1016/j.apradiso.2006.08.009
Elschot M, Vermolen BJ, Lam MG, de Keizer B, van den Bosch MA, de Jong HW (2013) Quantitative comparison of PET and Bremsstrahlung SPECT for imaging the in vivo yttrium-90 microsphere distribution after liver radioembolization. PLoS ONE. https://doi.org/10.1371/journal.pone.0055742
Kao YH, Tan EH, Ng CE, Goh SW (2011) Yttrium-90 time-of-flight PET/CT is superior to Bremsstrahlung SPECT/CT for postradioembolization imaging of microsphere biodistribution. Clin Nucl Med 36(12):e186–e187. https://doi.org/10.1097/RLU.0b013e31821c9a11
Kao YH, Tan EH, Lim KY, Ng CE, Goh SW (2012) Yttrium-90 internal pair production imaging using first generation PET/CT provides high-resolution images for qualitative diagnostic purposes. Br J Radiol 85(1015):1018–1019. https://doi.org/10.1259/bjr/33524085
Lhommel R, Goffette P, Van den Eynde M, Jamar F, Pauwels S, Bilbao JI, Walrand S (2009) Yttrium-90 TOF PET scan demonstrates high-resolution biodistribution after liver SIRT. Eur J Nucl Med Mol Imaging 36(10):1696. https://doi.org/10.1007/s00259-009-1210-1
Padia SA, Alessio A, Kwan SW, Lewis DH, Vaidya S, Minoshima S (2013) Comparison of positron emission tomography and bremsstrahlung imaging to detect particle distribution in patients undergoing yttrium-90 radioembolization for large hepatocellular carcinomas or associated portal vein thrombosis. J Vasc Interv Radiol 24(8):1147–1153. https://doi.org/10.1016/j.jvir.2013.04.018
Knešaurek K, Tuli A, Pasik SD, Heiba S, Kostakoglu L (2018) Quantitative comparison of pre-therapy 99mTc-macroaggregated albumin SPECT/CT and post-therapy PET/MR studies of patients who have received intra-arterial radioembolization therapy with 90Y microspheres. Eur J Radiol 109:57–61. https://doi.org/10.1016/j.ejrad.2018.10.015
Richetta E, Pasquino M, Poli M, Cutaia C, Valero C, Tabone M, Paradisi BP, Pacilio M, Pellerito RE, Stasi M (2019) PET-CT post therapy dosimetry in radioembolization with resin 90Y microspheres: comparison with pre-treatment SPECT-CT 99mTc-MAA results. Phys Med 64:16–23. https://doi.org/10.1016/j.ejmp.2019.05.025
Jadoul A, Bernard C, Lovinfosse P, Gérard L, Lilet H, Cornet O, Hustinx R (2020) Comparative dosimetry between 99mTc-MAA SPECT/CT and 90Y PET/CT in primary and metastatic liver tumors. Eur J Nucl Med Mol Imaging 47(4):828–837. https://doi.org/10.1007/s00259-019-04465-7
Ho CL, Chen S, Cheung SK, Leung YL, Cheng KC, Wong KN, Wong YH, Leung TWT (2018) Radioembolization with 90Y glass microspheres for hepatocellular carcinoma: significance of pretreatment 11C-acetate and 18F-FDG PET/CT and posttreatment 90Y PET/CT in individualized dose prescription. Eur J Nucl Med Mol Imaging 45(12):2110–2121. https://doi.org/10.1007/s00259-018-4064-6
Kuo JC, Tazbirkova A, Allen R, Kosmider S, Gibbs P, Yip D (2014) Serious hepatic complications of selective internal radiation therapy with yttrium-90 microsphere radioembolization for unresectable liver tumors. Asia Pac J Clin Oncol 10(3):266–272. https://doi.org/10.1111/ajco.12229
Currie BM, Hoteit MA, Ben-Josef E, Nadolski GJ, Soulen MC (2019) Radioembolization-induced chronic hepatotoxicity: a single-center cohort analysis. J Vasc Interv Radiol 30(12):1915–1923. https://doi.org/10.1016/j.jvir.2019.06.003
Benson AB 3rd, D’Angelica MI, Abbott DE, Abrams TA, Alberts SR, Saenz DA, Are C, Brown DB, Chang DT, Covey AM, Hawkins W, Iyer R, Jacob R, Karachristos A, Kelley RK, Kim R, Palta M, Park JO, Sahai V, Schefter T, Schmidt C, Sicklick JK, Singh G, Sohal D, Stein S, Tian GG, Vauthey JN, Venook AP, Zhu AX, Hoffmann KG, Darlow S (2017) NCCN Guidelines Insights: Hepatobiliary Cancers Version1.2017. J Natl Compr Canc Netw 15(5):563–573. https://doi.org/10.6004/jnccn.2017.0059
Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, Schirmacher P, Verslype C, Zech CJ, Arnold D, Martinelli E (2019) Correction to “Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up.” Ann Oncol 30(5):871–873. https://doi.org/10.1093/annonc/mdy510 (Epub 2019 Dec 4. Erratum for: Ann Oncol. 2018 Oct 1;29(Suppl 4):iv238-iv255. PMID: 31987361.)
Llovet JM, Finn RS (2018) Negative phase 3 study of 90Y microspheres versus sorafenib in HCC. Lancet Oncol 19(2):e69. https://doi.org/10.1016/S1470-2045(18)30025-1
Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, Sibert A, Bouattour M, Lebtahi R, Allaham W, Barraud H, Laurent V, Mathias E, Bronowicki JP, Tasu JP, Perdrisot R, Silvain C, Gerolami R, Mundler O, Seitz JF, Vidal V, Aubé C, Oberti F, Couturier O, Brenot-Rossi I, Raoul JL, Sarran A, Costentin C, Itti E, Luciani A, Adam R, Lewin M, Samuel D, Ronot M, Dinut A, Castera L, Chatellier G, SARAH Trial Group (2017) Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 18(12):1624–1636. https://doi.org/10.1016/S1470-2045(17)30683-6
Smits ML, Elschot M, van den Bosch MA, van de Maat GH, van het Schip AD, Zonnenberg BA, Seevinck PR, Verkooijen HM, Bakker CJ, de Jong HW, Lam MG, Nijsen JF (2013) In vivo dosimetry based on SPECT and MR imaging of 166Ho-microspheres for treatment of liver malignancies. J Nucl Med 54(12):2093–2100. https://doi.org/10.2967/jnumed.113.119768
Nayak D, Lahiri S (1999) Application of radioisotopes in the field of nuclear medicine: I. Lanthanide series elements. J Radioanal Nucl Chem 242(2):423–432
Turner JH, Claringbold PG, Klemp PF, Cameron PJ, Martindale AA, Glancy RJ, Norman PE, Hetherington EL, Najdovski L, Lambrecht RM (1994) 166Ho-microsphere liver radiotherapy: a preclinical SPECT dosimetry study in the pig. Nucl Med Commun 15(7):545–553
Seevinck PR, Seppenwoolde JH, de Wit TC, Nijsen JF, Beekman FJ, van Het Schip AD, Bakker CJ (2007) Factors affecting the sensitivity and detection limits of MRI, CT, and SPECT for multimodal diagnostic and therapeutic agents. Anticancer Agents Med Chem 7(3):317–334. https://doi.org/10.2174/187152007780618153
Arranja AG, Hennink WE, Denkova AG, Hendrikx RWA, Nijsen JFW (2018) Radioactive holmium phosphate microspheres for cancer treatment. Int J Pharm 548(1):73–81. https://doi.org/10.1016/j.ijpharm.2018.06.036
Bult W, Seevinck PR, Krijger GC, Visser T, Kroon-Batenburg LM, Bakker CJ, Hennink WE, van het Schip AD, Nijsen JF (2009) Microspheres with ultrahigh holmium content for radioablation of malignancies. Pharm Res 26(6):1371–1378. https://doi.org/10.1007/s11095-009-9848-8
Elschot M, Nijsen JF, Lam MG, Smits ML, Prince JF, Viergever MA, van den Bosch MA, Zonnenberg BA, de Jong HW (2014) (99m)Tc-MAA overestimates the absorbed dose to the lungs in radioembolization: a quantitative evaluation in patients treated with 166Ho-microspheres. Eur J Nucl Med Mol Imaging 41(10):1965–1975. https://doi.org/10.1007/s00259-014-2784-9
Prince JF, van den Bosch MAAJ, Nijsen JFW, Smits MLJ, van den Hoven AF, Nikolakopoulos S, Wessels FJ, Bruijnen RCG, Braat MNGJA, Zonnenberg BA, Lam MGEH. Efficacy of radioembolization with 166Ho-Microspheres in salvage patients with liver metastases: a phase 2 study. J Nucl Med 2018;59(4):582–588. https://doi.org/10.2967/jnumed.117.197194.
Prince JF, van Rooij R, Bol GH, de Jong HW, van den Bosch MA, Lam MG (2015) Safety of a scout dose preceding hepatic radioembolization with 166Ho microspheres. J Nucl Med 56(6):817–823. https://doi.org/10.2967/jnumed.115.155564
Braat AJAT, Prince JF, van Rooij R, Bruijnen RCG, van den Bosch MAAJ, Lam MGEH (2018) Safety analysis of holmium-166 microsphere scout dose imaging during radioembolisation work-up: a cohort study. Eur Radiol 28(3):920–928. https://doi.org/10.1007/s00330-017-4998-2
van Roekel C, van den Hoven AF, Bastiaannet R, Bruijnen RCG, Braat AJAT, de Keizer B, Lam MGEH, Smits MLJ (2021) Use of an anti-reflux catheter to improve tumor targeting for holmium-166 radioembolization-a prospective, within-patient randomized study. Eur J Nucl Med Mol Imaging 48(5):1658–1668. https://doi.org/10.1007/s00259-020-05079-0
Smits ML, Nijsen JF, van den Bosch MA, Lam MG, Vente MA, Mali WP, van Het Schip AD, Zonnenberg BA (2012) Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): a phase 1, dose-escalation study. Lancet Oncol 13(10):1025–1034. https://doi.org/10.1016/S1470-2045(12)70334-0 (Epub 2012 Aug 22. Erratum in: Lancet Oncol. 2012 Nov;13(11):e464. PMID: 22920685.)
Braat AJAT, Bruijnen RCG, van Rooij R, Braat MNGJA, Wessels FJ, van Leeuwaarde RS, van Treijen MJC, de Herder WW, Hofland J, Tesselaar MET, de Jong HWAM, Lam MGEH. Additional holmium-166 radioembolisation after lutetium-177-dotatate in patients with neuroendocrine tumour liver metastases (HEPAR PLuS): a single-centre, single-arm, open-label, phase 2 study. Lancet Oncol 2020;21(4):561–570. https://doi.org/10.1016/S1470-2045(20)30027-9.
Braat AJAT, Kwekkeboom DJ, Kam BLR, Teunissen JJM, de Herder WW, Dreijerink KMA, van Rooij R, Krijger GC, de Jong HWAM, van den Bosch MAAJ, Lam MGEH (2018) Additional hepatic 166Ho-radioembolization in patients with neuroendocrine tumours treated with 177Lu-DOTATATE; a single center, interventional, non-randomized, non-comparative, open label, phase II study (HEPAR PLUS trial). BMC Gastroenterol 18(1):84. https://doi.org/10.1186/s12876-018-0817-8.
Cullom SJ (1987) Quantitative imaging of bremsstrahlung radiation from P-32 in nuclear medicine, University of South Florida.
Balachandran S, McGuire L, Flanigan S, Shah H, Boyd CM (1985) Bremsstrahlung imaging after 32P treatment for residual suprasellar cyst. Int J Nucl Med Biol 12(3):215–221. https://doi.org/10.1016/0047-0740(85)90028-2
Clarke LP, Cullom SJ, Shaw R, Reece C, Penney BC, King MA, Silbiger M (1992) Bremsstrahlung imaging using the gamma camera: factors affecting attenuation. J Nucl Med 33(1):161–166
Pirayesh E, Amoui M, Akhlaghpoor S, Tolooee S, Khorrami M, Poorbeigi H, Sheibani S, Assadi M (2014) Technical considerations of phosphorous-32 Bremsstrahlung SPECT imaging after radioembolization of hepatic tumors: a clinical assessment with a review of imaging parameters. Radiol Res Pract. https://doi.org/10.1155/2014/407158
Pillai MR, Dash A, Knapp FF Jr (2012) Rhenium-188: availability from the (188)W/(188)Re generator and status of current applications. Curr Radiopharm 5(3):228–243. https://doi.org/10.2174/1874471011205030228
Häfeli UO, Casillas S, Dietz DW, Pauer GJ, Rybicki LA, Conzone SD, Day DE (1999) Hepatic tumor radioembolization in a rat model using radioactive rhenium (186Re/188Re) glass microspheres. Int J Radiat Oncol Biol Phys 44(1):189–199. https://doi.org/10.1016/s0360-3016(98)00554-9
Singh B (2002) Nuclear data sheets for A=188. Nucl Data Sheets 95:387–541. https://doi.org/10.1006/ndsh.2002.0005
Lambert B, Bacher K, Defreyne L, Gemmel F, Van Vlierberghe H, Jeong JM, Dierckx RA, Van de Wiele C, Thierens H, De Vos F (2005) 188Re-HDD/lipiodol therapy for hepatocellular carcinoma: a phase I clinical trial. J Nucl Med 46(1):60–66
De La Vega JC, Esquinas PL, Rodríguez-Rodríguez C, Bokharaei M, Moskalev I, Liu D, Saatchi K, Häfeli UO (2019) Radioembolization of hepatocellular carcinoma with built-in dosimetry: first in vivo results with uniformly-sized, biodegradable microspheres labeled with 188Re. Theranostics 9(3):868–883. https://doi.org/10.7150/thno.29381
Pillai MR, Chakraborty S, Das T, Venkatesh M, Ramamoorthy N (2003) Production logistics of 177Lu for radionuclide therapy. Appl Radiat Isot 59(2–3):109–118. https://doi.org/10.1016/s0969-8043(03)00158-1
Chakraborty S, Das T, Sarma HD, Venkatesh M, Banerjee S (2008) Preparation and preliminary studies on 177Lu-labeled hydroxyapatite particles for possible use in the therapy of liver cancer. Nucl Med Biol 35(5):589–597. https://doi.org/10.1016/j.nucmedbio.2008.03.003
Jadidan A, Anwari M, Riana A, Sari Y, Hardiansyah D (2021) Biodistribution study of lutetium hydroxyapatite (177Lu-HA) for liver cancer therapy. AIP Conf Proc. https://doi.org/10.1063/5.0047910
Hamiditabar M, Ali M, Bolek L, Vahdati G, Tworowska I, Delpassand ES (2017) Safety and effectiveness of 177Lu-DOTATATE peptide receptor radionuclide therapy after regional hepatic embolization in patients with somatostatin-expressing neuroendocrine tumors. Clin Nucl Med 42(11):822–828. https://doi.org/10.1097/RLU.0000000000001818
Ebbers SC, Braat AJAT, Moelker A, Stokkel MPM, Lam MGEH, Barentsz MW (2020) Intra-arterial versus standard intravenous administration of lutetium-177-DOTA-octreotate in patients with NET liver metastases: study protocol for a multicenter, randomized controlled trial (LUTIA trial). Trials 21(1):141. https://doi.org/10.1186/s13063-019-3888-0
Backhaus P, Noto B, Avramovic N, Grubert LS, Huss S, Bögemann M, Stegger L, Weckesser M, Schäfers M, Rahbar K (2018) Targeting PSMA by radioligands in non-prostate disease-current status and future perspectives. Eur J Nucl Med Mol Imaging 45(5):860–877. https://doi.org/10.1007/s00259-017-3922-y
Salas Fragomeni RA, Amir T, Sheikhbahaei S, Harvey SC, Javadi MS, Solnes LB, Kiess AP, Allaf ME, Pomper MG, Gorin MA, Rowe SP (2018) Imaging of nonprostate cancers using PSMA-targeted radiotracers: rationale, current state of the field, and a call to arms. J Nucl Med 59(6):871–877. https://doi.org/10.2967/jnumed.117.203570
Malik D, Sood A, Mittal BR, Singh H, Basher RK, Shukla J, Bhattacharya A, Singh SK (2018) Indian J Nucl Med 33(4):317–325. https://doi.org/10.4103/ijnm.IJNM_81_18
Jafari E, Ahmadzadehfar H, Dadgar H, Assadi M (2020) An overview on prostate-specific membrane antigen uptake in malignancies other than prostate cancer: a pictorial essay. World J Nucl Med 19(3):260–265. https://doi.org/10.4103/wjnm.WJNM_78_19
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C (1997) Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 3(1):81–85
Kratochwil C, Afshar-Oromieh A, Kopka K, Haberkorn U, Giesel FL (2016) Current status of prostate-specific membrane antigen targeting in nuclear medicine: clinical translation of chelator containing prostate-specific membrane antigen ligands into diagnostics and therapy for prostate cancer. Semin Nucl Med 46(5):405–418. https://doi.org/10.1053/j.semnuclmed.2016.04.004
Tolkach Y, Goltz D, Kremer A, Ahmadzadehfar H, Bergheim D, Essler M, Lam M, de Keizer B, Fischer HP, Kristiansen G (2019) Prostate-specific membrane antigen expression in hepatocellular carcinoma: potential use for prognosis and diagnostic imaging. Oncotarget 10(41):4149–4160. https://doi.org/10.18632/oncotarget.27024
Kuyumcu S, Has-Simsek D, Iliaz R, Sanli Y, Buyukkaya F, Akyuz F, Turkmen C (2019) Evidence of prostate-specific membrane antigen expression in hepatocellular carcinoma using 68Ga-PSMA PET/CT. Clin Nucl Med 44(9):702–706. https://doi.org/10.1097/RLU.0000000000002701
Bertagna F, Bertoli M, Bosio G, Giorgio B, Sadeghi R, Giubbini R, Treglia G (2014) Diagnostic role of radiolabelled choline PET or PET/CT in hepatocellular carcinoma: a systematic review and meta-analysis. Hep Intl 8:493–500. https://doi.org/10.1007/s12072-014-9566-0
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, Baum RP, Kulkarni HR, Schmidt M, Drzezga A, Bartenstein P, Pfestroff A, Luster M, Lützen U, Marx M, Prasad V, Brenner W, Heinzel A, Mottaghy FM, Ruf J, Meyer PT, Heuschkel M, Eveslage M, Bögemann M, Fendler WP, Krause BJ (2017) German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med 58(1):85–90. https://doi.org/10.2967/jnumed.116.183194
Seifert R, Kessel K, Boegemann M, Köhler M, Roll W, Stegger L, Weckesser M, Rahbar K (2020) Additional local therapy for liver metastases in patients with metastatic castration-resistant prostate cancer receiving systemic psma-targeted therapy. J Nucl Med 61(5):723–728. https://doi.org/10.2967/jnumed.119.233429
Sasikumar A, Joy A, Nanabala R, Pillai MR, Thomas B, Vikraman KR (2016) (68)Ga-PSMA PET/CT imaging in primary hepatocellular carcinoma. Eur J Nucl Med Mol Imaging 43(4):795–796. https://doi.org/10.1007/s00259-015-3297-x
Alipour R, Gupta S, Trethewey S (2017) 68Ga-PSMA uptake in combined hepatocellular cholangiocarcinoma with skeletal metastases. Clin Nucl Med 42(10):e452–e453. https://doi.org/10.1097/RLU.0000000000001771
Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG, Walenkamp AM (2013) A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer 49(1):219–230. https://doi.org/10.1016/j.ejca.2012.05.005
Walenkamp AME, Lapa C, Herrmann K, Wester HJ (2017) CXCR4 ligands: the next big hit? J Nucl Med 58(Suppl 2):77S-82S. https://doi.org/10.2967/jnumed.116.186874
Jeng KS, Jeng CJ, Jeng WJ, Chang CF, Sheen IS (2017) Role of C-X-C chemokine ligand 12/C-X-C chemokine receptor 4 in the progression of hepatocellular carcinoma. Oncol Lett 14(2):1905–1910. https://doi.org/10.3892/ol.2017.6396
Chatterjee S, Behnam Azad B, Nimmagadda S (2014) The intricate role of CXCR4 in cancer. Adv Cancer Res 124:31–82. https://doi.org/10.1016/B978-0-12-411638-2.00002-1.
Zhao H, Guo L, Zhao H, Zhao J, Weng H, Zhao B (2015) CXCR4 over-expression and survival in cancer: a system review and meta-analysis. Oncotarget 6(7):5022–5040. https://doi.org/10.18632/oncotarget.3217.
Schottelius M, Osl T, Poschenrieder A, Hoffmann F, Beykan S, Hänscheid H, Schirbel A, Buck AK, Kropf S, Schwaiger M, Keller U, Lassmann M, Wester HJ (2017) [177Lu]pentixather: comprehensive preclinical characterization of a first CXCR4-directed endoradiotherapeutic agent. Theranostics 7(9):2350–2362. https://doi.org/10.7150/thno.19119.
Schottelius M, Herrmann K, Lapa C (2021) In vivo targeting of CXCR4-new horizons. Cancers (Basel) 13(23):5920. https://doi.org/10.3390/cancers13235920.
Maurer S, Herhaus P, Lippenmeyer R, Hänscheid H, Kircher M, Schirbel A, Maurer HC, Buck AK, Wester HJ, Einsele H, Grigoleit GU, Keller U, Lapa C (2019) Side effects of CXC-chemokine receptor 4-directed endoradiotherapy with pentixather before hematopoietic stem cell transplantation. J Nucl Med 60(10):1399–1405. https://doi.org/10.2967/jnumed.118.223420
Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC (2016) Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res 18(1):84. https://doi.org/10.1186/s13058-016-0740-2.
Altmann A, Haberkorn U, Siveke J (2021) The latest developments in imaging of fibroblast activation protein. J Nucl Med 62(2):160–167. https://doi.org/10.2967/jnumed.120.244806
Bu L, Baba H, Yoshida N, Miyake K, Yasuda T, Uchihara T, Tan P, Ishimoto T (2019) Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene 38(25):4887–4901. https://doi.org/10.1038/s41388-019-0765-y
Zi F, He J, He D, Li Y, Yang L, Cai Z (2015) Fibroblast activation protein α in tumor microenvironment: recent progression and implications (review). Mol Med Rep 11(5):3203–3211. https://doi.org/10.3892/mmr.2015.3197
Scanlan MJ, Raj BK, Calvo B, Garin-Chesa P, Sanz-Moncasi MP, Healey JH, Old LJ, Rettig WJ (1994) Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci USA 91(12):5657–5661. https://doi.org/10.1073/pnas.91.12.5657
Jansen K, Heirbaut L, Verkerk R, Cheng JD, Joossens J, Cos P, Maes L, Lambeir AM, De Meester I, Augustyns K, Van der Veken P (2014) Extended structure-activity relationship and pharmacokinetic investigation of (4-quinolinoyl)glycyl-2-cyanopyrrolidine inhibitors of fibroblast activation protein (FAP). J Med Chem 57(7):3053–3074. https://doi.org/10.1021/jm500031w
Zimmerman C, Babich JW, Joyal J, Marquis J, Wang J (2010) Selective Seprase Inhibitors. Patent US 2010/0098633 A1. 2010 Apr 22
Loktev A, Lindner T, Mier W, Debus J, Altmann A, Jäger D, Giesel F, Kratochwil C, Barthe P, Roumestand C, Haberkorn U (2018) A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med 59(9):1423–1429. https://doi.org/10.2967/jnumed.118.210435
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, Adeberg S, Rathke H, Röhrich M, Winter H, Plinkert PK, Marme F, Lang M, Kauczor HU, Jäger D, Debus J, Haberkorn U, Giesel FL (2019) 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med 60(6):801–805. https://doi.org/10.2967/jnumed.119.227967
Giesel FL, Kratochwil C, Lindner T, Marschalek MM, Loktev A, Lehnert W, Debus J, Jäger D, Flechsig P, Altmann A, Mier W, Haberkorn U (2019) 68Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med 60(3):386–392. https://doi.org/10.2967/jnumed.118.215913
Shi X, Xing H, Yang X, Li F, Yao S, Zhang H, Zhao H, Hacker M, Huo L, Li X (2021) Fibroblast imaging of hepatic carcinoma with 68Ga-FAPI-04 PET/CT: a pilot study in patients with suspected hepatic nodules. Eur J Nucl Med Mol Imaging 48(1):196–203. https://doi.org/10.1007/s00259-020-04882-z
Zhao L, Gu J, Fu K, Lin Q, Chen H (2020) 68Ga-FAPI PET/CT in assessment of liver nodules in a cirrhotic patient. Clin Nucl Med 45(10):e430–e432. https://doi.org/10.1097/RLU.0000000000003015
Pang Y, Hao B, Shang Q, Sun L, Chen H (2020) Comparison of 68Ga-FAPI and 18F-FDG PET/CT in a patient with cholangiocellular carcinoma: a case report. Clin Nucl Med 45(7):566–567. https://doi.org/10.1097/RLU.0000000000003056
Guo W, Pang Y, Yao L, Zhao L, Fan C, Ke J, Guo P, Hao B, Fu H, Xie C, Lin Q, Wu H, Sun L, Chen H (2021) Imaging fibroblast activation protein in liver cancer: a single-center post hoc retrospective analysis to compare [68Ga]Ga-FAPI-04 PET/CT versus MRI and [18F]-FDG PET/CT. Eur J Nucl Med Mol Imaging 48(5):1604–1617. https://doi.org/10.1007/s00259-020-05095-0
Acknowledgements
No Acknowledgments
Author information
Authors and Affiliations
Contributions
MA: The idea for the article; NJ: The literature search and the first draft for manuscript; EJ: Data collection; HA, FM, SN, ARR, MA: The critically revised the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest:
No conflict of interest.
Ethics approval:
This study was approved by ethical standards of the responsible committee and was carried out in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The study was also approved by the Bushehr University of Medical Science Research Ethics Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jokar, N., Moradhaseli, F., Ahmadzadehfar, H. et al. Theranostic approach in liver cancer: an emerging paradigm to optimize personalized medicine. Clin Transl Imaging 11, 51–70 (2023). https://doi.org/10.1007/s40336-022-00525-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-022-00525-5