Opinion statement
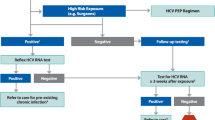
Occupational injuries and exposure to blood and body fluids continue to be commonplace in virtually every healthcare setting. Primary prevention of these injuries and exposure is the most important component of preventing occupational infection with any blood-borne pathogen. Should primary prevention fail, secondary prevention consists of post-exposure prophylaxis customized for the specific exposure. The 2013 U.S. Public Health Service recommendations for post-exposure prophylaxis following exposure to HIV include a “preferred” regimen of combined raltegravir, tenofovir, and emtricitabine, although these guidelines are now being continually updated on the CDC website. All healthcare workers started on HIV post-exposure prophylaxis should be re-evaluated within 72 hours, and follow-up HIV testing should occur at 6 weeks, 12 weeks, and from 4 to 6 months after exposure, depending on whether a fourth-generation immunoassay for HIV is used. The three intramuscular doses of hepatitis B vaccine that all healthcare workers should receive at the time of entry into patient care induce a protective antibody response in more than 90 % of healthy recipients. For individuals susceptible to HBV who are exposed to this virus, immunoprophylaxis consists of one dose of hepatitis B immune globulin (HBIG) and reinitiating the vaccine series for nonresponders who have not completed a second three-dose vaccine series. For persons who previously completed a second vaccine series but failed to respond, two doses of HBIG are preferred. The potential for occupational exposure to HCV is increasing, but no vaccine is currently available, and no prophylaxis is recommended. Highly active direct-acting anti-HCV antivirals that inhibit HCV protease, polymerase, and nonstructural protein 5A may eventually play a role in the management of such exposures, but are as yet untested in this setting. In addition, spontaneous clearance of HCV is common, acute HCV infection is highly curable, and early therapy results in high rates of eradication.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Panlilio AL, Orelien JH, Srivastava PU, Jagger J, Cohn RD, Cardo DM, et al. Estimate of the annual number of percutaneous injuries among hospital-based healthcare workers in the United States, 1997–1998. Infect Control Hosp Epidemiol. 2004;25:556–62.
Cardo DM, Culver DH, Ciesielski CA, Srivastava PU, Marcus R, Abiteboul D, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337:1485–90.
Centers for Disease Control and Prevention. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR 2013,62:1–19.
Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O'hUigin C, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801.
Corey KE, Mendez-Navarro J, Gorospe EC, Zheng H, Chung RT. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat. 2010;17:201–7.
Galbraith JW, Donnelly JP, Franco RA, Overton ET, Rodgers JB, Wang HE. National estimates of healthcare utilization by individuals with hepatitis C virus infection in the United States. Clin Infect Dis. 2014;59:755–64.
Blauvelt A, Glushakova S, Margolis LB. HIV-infected human Langerhans cells transmit infection to human lymphoid tissue ex vivo. AIDS. 2000;14:647–51.
Pope M, Gezelter S, Gallo N, Hoffman L, Steinman RM. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ cells. J Exp Med. 1995;182:2045–56.
Tsai CC, Emau P, Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–73.
Bottiger D, Johansson NG, Samuelsson B. al E. Prevention of simian immunodeficiency virus, SIVsm, or HIV-2 infection in cynomolgus monkeys by pre- and postexposure administration of BEA-005. AIDS. 1997;11:157–62.
Tsai CC, Emau P, Sun JC. al E. Post-exposure chemoprophylaxis (PECP) against SIV infection of macaques as a model for protection from HIV infection. J Med Primatol. 2000;29:248–58.
Pinto LA, Sullivan J, Berzofsky JA, Clerici M, Kessler HA, Landay AL, et al. ENV-specific cytotoxic T lymphocyte responses in HIV seronegative health care workers occupationally exposed to HIV-contaminated body fluids. J Clin Invest. 1995;96:867–76.
Clerici M, Levin JM, Kessler HA, Harris A, Berzofsky JA, Landay AL, et al. HIV-specific T-helper activity in seronegative health care workers exposed to contaminated blood. JAMA. 1994;271:42–6.
Henderson DK. Managing occupational risks for hepatitis C transmission in the health care setting. Clin Microbiol Rev. 2003;16:546–68.
Rehermann B, Bertoletti A. Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections. Hepatology 2014.This manuscript details current understanding of how patients' immune responses affect treatment outcomes for HBV and HCV infection as well as how immune responses are altered by different treatment regimens.
Beekmann SE, Henderson DK. Protection of healthcare workers from bloodborne pathogens. Curr Opin Infect Dis. 2005;18:331–6.
MacCannell T, Laramie AK, Gomaa A, Perz JF. Occupational exposure of health care personnel to hepatitis B and hepatitis C: prevention and surveillance strategies. Clin Liver Dis. 2010;14:23–36.
Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–8.
Corey KE, Servoss JC, Casson DR, Kim AY, Robbins GK, Franzini J, et al. Pilot study of postexposure prophylaxis for hepatitis C virus in healthcare workers. Infect Control Hosp Epidemiol. 2009;30:1000–5.
Das S, Shetty RK, Kumar A, Shridharan RN, Tatineni R, Chi G, et al. Monoclonal antibodies against hepatitis C genotype 3a virus like particle inhibit virus entry in cell culture system. PLoS One. 2013;8:e53619. This paper demonstrates that monoclonal antibodies directed against HCV envelope proteins can prevent viral entry into liver cells, thus blocking HCV replication and has clear implications concening the potential for immunprophylaxis.
Kuhar DT, Henderson DK, Struble KA, Heneine W, Thomas V, Cheever LW, et al. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875–92. This paper describe the most recently issued set of United States Public Health Service recommendations for postexposure prophyalixis for occupational HIV exposures.
Ciesielski CA, Metler RP. Duration of time between exposure and seroconversion in healthcare workers with occupationally acquired infection with human immunodeficiency virus. Am J Med. 1997;102:115–6.
Compliance with Ethics Guidelines
Conflict of Interest
David Henderson and Susan Beekmann have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on New Technologies and Advances in Infection Prevention
Rights and permissions
About this article
Cite this article
Beekmann, S.E., Henderson, D.K. Occupational Exposures among Healthcare Workers: New Methods for Prevention and Recommended Postexposure Prophylaxis for HIV and Hepatitis B and C. Curr Treat Options Infect Dis 7, 28–38 (2015). https://doi.org/10.1007/s40506-014-0036-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40506-014-0036-y