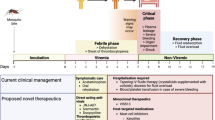
Opinion statement
Purpose of review
Ebola virus, a member of the Filoviridae family, is a causative agent of severe viral hemorrhagic fever in humans. Over the past 40 years, the virus has been linked to several high mortality outbreaks in Africa with the recent West African outbreak resulting in over 11,000 deaths. This review provides a summary of the status of the drug discovery and development process for therapeutics for Ebola virus disease, with a focus on the strategies being used and the challenges facing each stage of the process.
Recent findings
Despite the wealth of in vitro efficacy data, preclinical data in animal models, and human clinical data, no therapeutics have been approved for the treatment of Ebola virus disease. However, several promising candidates, such as ZMapp and GS-5734, have advanced into ongoing clinical trials.
Summary
The gravity of the 2014-2016 outbreak spurred a heightened effort to identify and develop new treatments for Ebola virus disease, including small molecules, immunotherapeutics, host factors, and clinical disease management options.
Disclaimer
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endoresed by the U.S. Army.
Similar content being viewed by others
Introduction
Drug development for Ebola virus (EBOV) has been in progress for several decades, primarily fueled by concerns about the potential use of the Category A agent as a bioweapon. However, the unprecedented magnitude and scale of the 2014–2016 outbreak in West Africa, combined with the potential spread to other corners of the world, led to a renewed focus on medical countermeasures for Ebola virus disease (EVD).
Ebola virus is a member of the Filoviridae family which includes the three genera Ebolavirus, Marburgvirus and Cuevavirus [1]. The genus Ebolavirus contains its eponymous member EBOV, in addition to four related species: Sudan virus, Tai Forest virus, Bundibugyo virus, and Reston virus [1]. Filoviruses are pleomorphic in shape and are encased in a lipid envelope. The negative-sense single-stranded RNA genome is approximately 19 kb in size, and consists of a linear, non-segmented RNA. The linear viral genome encodes for seven proteins: nucleoprotein (NP), polymerase cofactor VP35, matrix proteins VP40 and VP24, glycoprotein (GP), transcription activator VP30, and RNA-dependent RNA polymerase (L).
Humans are thought to be an “accidental” host for filoviruses as opposed to a natural reservoir, due to the high mortality rates associated with EVD outbreaks in humans. Serological data indicates that bats are likely a natural reservoir, or other species yet to be identified [2]. Transmission occurs through contact with bodily fluids of an infected patient or animal, either through direct inoculation such as a needlestick or exposure of broken skin and/or mucous membranes. While early cellular targets for infection are dendritic cells, monocytes, and macrophages, a variety of cells are infected by EBOV as the disease progresses [3]. EBOV infection of cells leads to dysregulation of the immune response including suppression of type I interferon responses due to the action of viral proteins such as VP24 and VP35 [4•]. Conversely, massive release of proinflammatory cytokines and chemokines, termed “cytokine storm,” is also a hallmark of EBOV infection and likely contributes to inflammation and other disease manifestations [4•, 5].
Ebola virus is a causative agent of viral hemorrhagic fever which is associated with mortality rates as high as 90% in humans [6, 7]. Initial clinical signs of EVD include non-specific symptoms such as fever, malaise, and gastrointestinal involvement, followed by a rapid progression to shock, organ failure, and death [8•]. Despite being a causative agent of viral hemorrhagic fever, hemorrhage is often only present in a fraction of cases. Some patients may present with petechiae and/or a maculopapular rash. Common laboratory findings in EVD include lymphopenia, anemia, elevated liver enzymes, and evidence of coagulopathy including thrombocytopenia and high levels of D-dimers [8•].
Although past EVD outbreaks have relied extensively on supportive care measures such as fluid and electrolyte replacement, a number of experimental therapeutics were evaluated in clinical trials and under compassionate use protocols during the 2014–2016 outbreak. This review focuses on the status of EVD therapeutics at the three main stages of the drug development pathway (discovery, preclinical, and clinical) with a view towards the fundamental principles of pharmacokinetic/pharmacodynamic (PK/PD) relationships such as (a) interaction of the drug with the target, (b) drug exposure at the site of action, and (c) expression of pharmacological activity in the target tissue [9]. This article does not include a discussion of vaccine development efforts and candidates, which have been extensively discussed in other reviews [10].
Discovery of Compounds for the Treatment of Ebola Virus: Early Steps
Biological Targets for the Treatment of EVD
Therapeutic development requires the identification of proteins, RNA, or other biological components that will make suitable drug targets. Drug targets, which are identified and validated through a combination of biochemical, genetic, structural, and computational strategies, are generally derived from either the host or pathogen. As such, therapeutic strategies to fight EBOV fall into four main categories: direct targeting of the virus, modulation of host factors, modulation of the immune response, and management of clinical disease.
One of the most popular strategies for EBOV therapeutics is antivirals that directly target critical stages in the viral life cycle such as binding and/or entry of the virus into host cells, viral replication, packaging, or release of viral progeny from target cells. Small molecules, antisense therapies, and immunotherapeutics comprise the diverse list of EBOV antiviral compounds. A disproportionate number of the most advanced therapeutics currently under evaluation are small molecules directed against the RNA-dependent RNA polymerase L required for viral replication. These include the nucleoside analogs BCX4430 [11, 12], GS-5734 [13••], and favipiravir (T-705) [14, 15] which are intracellularly converted to the active nucleoside triphosphate (or nucleotide). The two primary classes of antisense therapies are small-interfering RNAs (siRNAs), which promote degradation of mRNA transcripts, and phosphorodiamidate morpholino oligomers (PMOs) that interfere with translation [16]. TKM-100802 (TKM-Ebola) and its derivative TKM-130803, which was designed for improved targeting of the West African strain of EBOV, are combinations of three siRNAs that hit multiple viral targets (L, VP35, and VP24). AVI-7537 and AVI-6002 (a combination that includes AVI-7537 and AVI-7539) are PMOs that target VP24 and VP24/VP35, respectively. In vitro investigation of other viral proteins such as VP35, VP24, and VP40 as potential new targets for EBOV drugs is currently underway. Direct antivirals also include many of the immunotherapeutics under development that bind to the virus and prevent entry. As the only surface expressed protein of EBOV, GP is a common target of such therapeutic antibodies including the ZMapp antibody cocktail, monoclonal, and polyclonal antibodies. The ZMapp cocktail is comprised of three monoclonal chimeric antibodies with neutralizing activity that target the GP base and glycan cap [17]. Other immunotherapeutics that target GP include lectins such as mannose binding lectin (MBL) which have shown efficacy in in vitro and rodent models [18].
Host factor modulators have gained recent interest in the EBOV field. Like many other viruses with limited genomes, EBOV utilizes host proteins to gain entry and undergo replication. Several host proteins involved in EBOV entry have been tested, including cathepsins, Niemann-Pick C1 (NPC1), and T-cell immunoglobulin and mucin 1 (TIM-1). Cathepsins such as CatB and CatL are cysteine proteases in the endosome that cleave EBOV GP prior to fusion and entry. Although protease and cathepsin inhibitors have shown efficacy in vitro against EBOV [19, 20], it remains unclear whether specific targeting of cathepsins could be used therapeutically due to potential compensatory mechanisms. The cholesterol transport protein Niemann-Pick C1 protein (NPC1) has been shown to bind GP following cathepsin-mediated cleavage. Two small molecules, MBX2254 and MBX2270, are thought to inhibit binding of EBOV GP to NPC1, thus inhibiting infection in in vitro assays [21]. TIM-1 has been shown to bind to GP, thus serving as a receptor for EBOV and other filoviruses [22]. Inhibition of EBOV infection occurred following treatment of cells with the TIM-1 antibody ARD5, suggesting that TIM-1 may be a worthwhile target for EBOV therapeutics [22].
Modulation of the immune system is another strategy that has been investigated for the treatment of EVD. Immunomodulators for EBOV infection, including cytokines, chemokines, and other proteins, may enhance the immune response, thereby promoting viral clearance. They may alternatively dampen undesirable immune responses such as the overwhelming inflammatory cytokine release associated with EBOV. As several EBOV proteins are known to subvert the interferon response, treatment with type I interferons (−α and -β) have been investigated as potential EBOV therapies. The combination of adenovirus-vectored interferon-α with ZMab antibody has been investigated in post-exposure studies in NHPs with success in improving survival and reducing viral loads [23]. Similar treatment of NHPs with interferon-β resulted in extended time to death [24].
Management therapies aim to treat the clinical manifestations of EVD which include coagulation abnormalities and hemorrhagic manifestations. Anticoagulants such as recombinant human activated protein C (rhAPC) and recombinant nematode anticoagulant protein c2 (rNAPc2) that affect the coagulation pathway have been investigated [25, 26]. FX06, a fibrin-derived peptide under development to treat vascular leak syndrome, was administered to patient during the outbreak in an effort to stem vascular leakage [27].
In Vitro Efficacy Data for EBOV Therapeutics (Interaction of the Drug With the Target)
Hit identification for new EBOV therapeutics has occurred mainly through in vitro screening with cell-culture based assays, many of which have high-throughput capability to facilitate the screening of large compound libraries. Those most commonly used are pseudotyped-virus assays that can be performed in biosafety level (BSL)-2 facilities, and replication assays with infectious EBOV which are limited to BSL-4 laboratories. In addition to overcoming biosafety restrictions, pseudotyped-virus assays, in which EBOV GP is expressed on a viral backbone such as HIV or VSV, are useful in determining if the drug inhibits the viral entry process. This information may assist with narrowing down the target or mechanism of action for compounds identified in phenotypic screens. The readouts for these assays include reduction in cytopathic effects or reduction in viral replication as measured by PCR or fluorescent imaging. Table 1 provides a list of compounds and their level of efficacy, reported as either IC50 or EC50, against EBOV in in vitro assays, although strictly speaking these data all appear to be concentrations of drug that give a half-maximal response.
As a variety of assays have been used to assess the in vitro efficacy of compounds against EBOV, it can be difficult to compare results across different platforms and techniques. This is readily apparent in instances where a compound tested in parallel against both pseudotyped and wild-type virus generates different EC50/IC50 values for each assay. The discrepancy may be due to differences in GP expression between the two types of viral particles [21].
In addition to the screening of novel compound libraries and lead optimization campaigns, marketed drugs originally intended for other indications such as cancer, depression, malaria and bacterial infections have also been screened and identified as having anti-EBOV activity. These include toremiphene, clomipheme, amodiaquine, azithromycin, and chloroquine (Table 1), most of which have been classified as cationic amphiphilic drugs (CADs) [32•]. However, the mechanism of action of some of these drugs against EBOV is unclear. Screening on behalf of drug repurposing efforts has also identified hits against other viral targets such as VP24 [64].
Pharmacokinetics and Tolerability of EBOV Therapeutics (Adequate Drug Exposure at the Site of Action)
Table 1 includes PK and tolerability data, both of which are critical for the selection of compounds capable of providing in vivo efficacy. Drug tolerability is especially important for preclinical EBOV therapeutic studies since survival is viewed as a key efficacy endpoint. As a rule of thumb, free drug concentrations at the target site should be sustained above the EBOV EC50 values when administered at a dose below the maximum tolerated dose (MTD). This brings up a key question of how the “target site” is defined. Researchers typically compare plasma PK curves (converted to unbound plasma concentrations) to the corresponding EBOV EC50 (or EC90) value to design a dosing regimen and/or determine if a compound is capable of providing enough exposure to merit efficacy testing. However, EBOV is present in macrophages, dendritic cells, and monocytes by Day 2 post-infection, and in fibroblasts, Kupffer cells, polymorphonuclear cells, endothelial cells, adrenal cortical cells, tubular epithelium, stromal cells, stromal stellate cells, tonsillar epithelium, and hepatocytes—and practically every tissue—by Day 5 [65, 66]. Moreover, EBOV has been found to persist in the semen and eyes of EBOV survivors [67]. Therefore, effective EBOV therapeutics should not be exquisitely selective for any particular cell or tissue, but rather may need to be well-distributed (e.g., high volume of distribution, V d ). Researchers with drugs at early stages of development often face the dilemma of whether to use plasma alone to assess adequate drug exposure, or to also invest in more rigorous PK studies that involve defining the cell/tissue distribution of the drug. This is even more critical for nucleosides since the plasma half-life of the nucleoside is often very short due to rapid permeation into cells followed by intracellular conversion to their corresponding nucleoside triphosphate (TP, the active drug) where they can persist (longer half-life). For example, in the mouse BCX4430 has a 10 min half-life in plasma, but its TP in liver has a 4.3 h half-life. This conversion was found to be greater in mouse hepatocytes compared to human (Table 1), highlighting the importance of interspecies translation. Noteworthy is that the efficacious dose in the mouse (150 mg/kg, IM) corresponded to liver TP levels that were ×2.5 above the EBOV EC50. For another nucleoside, GS-5734, an effective dose that achieved 100% protection in NHPs (10 mg/kg) corresponded with rapid uptake into monkey PBMCs and triphosphate levels persisting above the EBOV EC50 over a 24-h period.
For many of the drug repurposing efforts, the maximum unbound drug concentrations in human plasma at the highest FDA-approved doses are well below the EBOV EC50 values (e.g., CADs in Table 1). Where mouse PK data are available (chloroquine, toremiphene, azithromycin, sertraline), unbound plasma levels appear insufficient, with the exception of azithromycin (50 mg/kg, PO) where the mouse PPB is low [68]. It is surprising that no improvements to survival were observed in the mouse by oral administration of azithromycin, especially since adequate plasma and tissue exposures were likely achieved at the 100 mg/kg dose. This result suggests that the CAD mechanism is not an effective approach to treat EVD.
Preclinical Efficacy in Animal Models (Expression of Pharmacological Activity in the Target Tissue)
Following the identification of active leads and subsequent determination of their PK and tolerability, promising therapeutic compounds are advanced into preclinical studies focused on gathering data that will inform future clinical trial design and development of a promising therapeutic candidate into a safe and marketable product. This includes evaluation in animal models of the disease or condition to assess in vivo efficacy. Therapeutics in development for the treatment of EVD are tested in lethal EBOV models in mice, guinea pigs, and/or nonhuman primates (NHPs). Due to the cost, lower risk, and relative ease associated with rodent and small animal models, many therapeutics for EBOV are initially evaluated in mice and/or guinea pigs. However, wild-type EBOV is not lethal in adult immunocompetent mice or guinea pigs; both models require the use of adapted virus generated through repeated serial passage. Both models also lack some of the salient features of EVD in humans, including alterations in immune cell populations for guinea pigs and hemorrhagic and coagulation abnormalities for mice. Efficacy in both the rodent and guinea pig models is measured in terms of survival, with reduction in weight loss and extended time to death serving as alternative endpoints. Rodent models are also particularly useful for testing and validating novel targets and mechanisms of action prior to performing more intensive NHP studies. For example, target validation studies reveal that viral replication and mortality in the mouse model are decreased following VP35 inhibition [69].
Candidates that demonstrate efficacy in the mouse or guinea pig models may advance into the NHP model, which is considered to be the most accurate surrogate for human EVD due to the fact that the clinical picture in NHPs is remarkably similar to humans in terms of hemorrhagic manifestations, coagulopathy, and pathology [70]. Efficacy in the NHP model is measured primarily by survival; however, reduction in viral load and delayed time to death have also been used. For example, treatment with interferon-β prolongs survival in EBOV-infected macaques [24]. However, efficacy in the rodent model does not always translate to the NHP model. This may be due in part to the fact that EBOV does not cause the same disease manifestations in mice as in NHPs. Alternatively, the threshold for achieving protection in a mouse may be lower than that in NHPs.
The choice of animal model is occasionally dependent on the limitations associated with the different species. For example, some host-modulating drugs cannot be evaluated in rodent models as the target may not be expressed in mice. Alternatively, some drugs may possess properties that render them unsuitable for evaluation in an animal model. For example, evaluation of brincidofovir in the NHP model can be challenging due to metabolism of the drug in primates. The conversion of brincidofovir to its active form is significantly less efficient in NHPs than other species including mice and humans, resulting in lower systemic exposure [71]. As such, efficacy data for brincidofovir will need to be generated from human clinical studies. Other host-modulating drugs are dependent on the inflammatory response for which the translation from mice to human can be questioned [72]. Table 1 includes relevant in vivo efficacy data for various EBOV therapeutics under development.
Clinical Trials and Observational Studies of EBOV Therapeutics
The 2014–2016 outbreak in West Africa motivated the initiation of multiple clinical trials for lead candidates that previously demonstrated efficacy against EBOV in animal models. Clinical trials are divided into three phases I, II, and III, which differ in their objectives and scope. The EBOV therapeutics in advanced development have only been evaluated in Phase I and Phase II trials for safety and efficacy, and due to the severity and urgency of the disease, but limited duration of the outbreak, clinical studies carried out during the outbreak have lacked proper controls and/or statistical power. More recent EBOV drug candidates such as GS-5734 and BCX4430 have completed Phase I trials and the former is currently being tested for its ability to reduce the viral load in the semen of male EBOV survivors (Table 2). AVI-6002 and AVI-7537 have completed Phase I trials but are not being developed by manufacturer despite preclinical efficacy data in three species and encouraging human data for safety, tolerability and PK.
Although many of the therapeutic options highlighted in the 2014–2016 outbreak had favorable Phase I outcomes, safety concerns have been identified for some products. One example is TKM-100802 which was evaluated in a Phase I trial in healthy individuals in January 2014. The trial was put on hold by the FDA due to safety concerns arising from the development of flu-like symptoms in treated individuals which was ultimately linked to cytokine release triggered by the action of the siRNA [85]. The hold was initially relaxed to allow for expanded access for EVD patients during the outbreak and was ultimately removed, enabling the study to resume at a lower dose than the one initially tested [85].
Phase II/III trials are designed to assess efficacy, with the gold standard being the randomized controlled trial in which patients are randomized to placebo or treatment arms. Although reduction in mortality is the most sought after endpoint in these trials, secondary endpoints such as reduction in viral loads are also measured. The objectives for the trial are outlined prior to its initiation; if early analysis of the trial data indicates that the objectives are not likely to be achieved, the trial may be terminated early on the basis of “futility.” Following successful restart and conclusion of its Phase I trial, TKM-130803 entered a single-arm Phase II trial in March 2015 in Sierra Leone [77]. The study was prematurely terminated due to the lack of efficacy data. Further development of TKM-Ebola has been suspended by the manufacturer (Tekmira press release 2015).
In order to demonstrate a statistically significant benefit for the therapeutic, clinical trials must be appropriately powered, requiring a minimum number of participants. Enrolling sufficient numbers of clinical trial participants is often challenging and may contribute to the success or failure of a study. Low enrollment can be due to factors such as cultural ideologies, inaccessibility of trial sites, and communication challenges. The PREVAIL II study, which evaluated ZMapp in a randomized controlled trial in Liberia, Guinea, Sierra Leone, and the USA, did not produce any statistically definitive conclusions concerning the efficacy of the treatment due to low enrollment numbers [82••]. Similarly, the Phase II trial for brincidofovir to assess safety, tolerability and efficacy was discontinued after 1 month due to a lack of enrollment [77]. Brincidofovir is not currently being advanced as an EBOV therapeutic candidate.
Statistical power is not the only key characteristic to a well-designed study. Favipiravir was tested for efficacy in 2014 in the JIKI trial (a multicenter proof-of-concept non-comparative trial conducted in four Ebola treatment centers in Guinea) which was heavily critiqued for its design: it was not randomized and relied on the use of historical controls [78]. Due to this, many found the results of the study to be difficult to reliably interpret. The current efficacy data for favipiravir is inconclusive and suggests that the drug may only be effective in treating patients with low to moderate levels of virus (CT values > than 20) [78]. Similarly, convalescent plasma was evaluated in a non-randomized, historically controlled Ebola-Tx trial in Guinea; it did not appear to demonstrate an improvement in survival [83]. The failure of some EBOV drugs to demonstrate efficacy in Phase II trials, as well as the occasional challenges in interpreting such clinical data, highlights the importance of well-designed studies. However, design of these trials may present ethical issues that are associated with high mortality pathogens such as EBOV. Randomized, controlled studies typically randomize participants to either an untreated or placebo control arm or a treated arm. However, it is considered unethical to provide no treatment for a high mortality pathogen such as EBOV. To remedy this concern, many studies have used supportive care as the control arm in an effort to assess whether the therapeutic under evaluation improves survival as compared to the current standard of care. Many of the drugs evaluated for efficacy in clinical trials have also been administered to EBOV-infected individuals under expanded access or emergency use authorization. In such cases, investigational drugs or drugs approved for other indications may be administered to critical patients. Several patients received brincidofovir during the outbreak under such circumstances, including one who received brincidofovir in combination with convalescent plasma and supportive care and survived [74]. ZMapp was also administered on a compassionate use basis to EVD patients, including several healthcare workers who survived. However, none of these treatments appeared to demonstrate statistically powered efficacy against EBOV. Part of the difficulty in interpreting such data is due to the fact that these patients often received more than one experimental treatment in combination. In such cases, it can be difficult to ascertain which drug was responsible for the therapeutic effect.
Challenges for EBOV Drug Discovery and Development
Although many options for the treatment of EVD have begun to enter the clinical trial pipeline, there is still no FDA-approved therapy. The cessation of the 2014–2016 outbreak has returned EBOV to its previous status as the causative agent of limited, sporadic outbreaks. Due to this, as well as the unpredictability of such outbreaks, Phase II/III clinical trials aimed at assessing efficacy are difficult at the present time. While it still remains possible to evaluate safety, dose ranges, and adverse events in Phase I trials, many early trials are typically conducted at research sites in the USA. However, the populations in which safety is initially evaluated are often distinct from those in which outbreaks occur. Due to genetic and immunological differences among different patient populations, care should be taken when interpreting Phase I safety data prior to collection of additional safety data in diverse populations in Phase III. In addition, the patient populations within the historic outbreak regions are susceptible to malaria which may present similar triggers for treatment (e.g., fever, headache, muscle pain, fatigue, diarrhea, vomiting), and anti-malarial co-medication may give rise to drug-drug interactions and potentially confound phase II/III results.
The use of the FDA Animal Rule as a potential mechanism for drug approval has been proposed for EBOV. The Animal Rule was developed for high-consequence pathogens for which efficacy studies or field trials in humans are not feasible or ethical. Approval under the Animal Rule requires that efficacy must be demonstrated in at least one animal model that accurately reflects human disease. Additionally, the mechanism of action of the drug must be characterized. Occasionally drugs have shown efficacy against EBOV without a true identified target or mechanism of action. One prominent example is brincidofovir which was investigated as a therapeutic for adenovirus, smallpox, and cytomegalovirus [86]. As its mechanism of action is against dsDNA viruses, it is currently unknown how the compound inhibits an RNA virus such as EBOV.
Some of the therapeutics shown to be efficacious present challenges for large-scale production and formulation. The antibodies that make up the ZMapp cocktail are manufactured in tobacco plants (Nicotiana benthamiana). As such, there are limitations to manufacturing the antibodies, with supplies of the drug an issue during the outbreak. To remedy this problem, the manufacturing of ZMapp in Chinese hamster ovary (CHO) cells, which has been successfully utilized for other antibodies, has been developed and investigated [87]. An ideal therapeutic candidate for EBOV can be rapidly synthesized in large quantities so it can be made readily available during an outbreak. Convalescent plasma/serum can be similarly difficult to obtain large quantities of as it requires that samples be obtained from infected individuals.
The sheer magnitude and speed of the 2014–2016 outbreak, coupled with the challenges with the drug discovery and development process, necessitated the exploration of alternative strategies. One of these options was the repurposing of drugs in an effort to accelerate the process to place an approved therapy at the disposal of those in need more quickly. Drug repurposing, or the investigation of approved drugs or compounds for new indications, has been a hotly contested issue in the field. The primary advantage of using repurposed drugs is that they have been previously approved by the FDA for other indications. FDA approval is accompanied by a known safety profile, including toxicity, pharmacokinetic, pharmacodynamics and dosing data. As such, repurposed drugs may be accelerated through the FDA approval process by bypassing Phase I, expediting the process by months to years. The use of repurposed drugs may also alleviate some of the logistical considerations and challenges with development of a new drug, including manufacturing and distribution. However, some critics of drug repurposing argue that while such efforts may cut down on the development timeline, any unfavorable data gained from additional clinical trials may be detrimental to the current approval status for these drugs. Additionally, it may be difficult to discern the mechanism of action against EBOV for these agents. Unlike direct antiviral targets or targets within the host that are known to be critical to the virus life cycle or immune response, many of these repurposed drugs target processes that on the surface are seemingly unrelated to EBOV infection. Experiments aimed at identifying the mechanism of action of these compounds may provide new insights into the EBOV life cycle.
Additional data that has emerged from the 2014–2016 outbreak has indicated that EBOV may persist in bodily fluids such as semen for months following recovery from infection [88, 89]. In some instances, virus persistence in the semen has been implicated in the sexual transmission of EBOV from survivors to their partners [90]. As such, the development of therapeutics that are capable of eliminating EBOV from bodily fluids has become a topic of investigation. The PREVAIL IV trial is currently underway to investigate the ability of GS-5734 to eliminate persistent viral RNA in semen of survivors (NCT02818582).
Conclusions
The drug discovery pipeline is a challenging and time-consuming process that is often plagued by high costs and high attrition rates, with very few of the numerous compounds evaluated in the early discovery stages ever making it to the clinic. The vast majority of the drug discovery efforts for Ebola, including those discussed here, have focused on EBOV Zaire. However, there is considerable interest in developing a therapeutic with broad-spectrum activity against other filoviruses such as Marburg virus and Sudan virus, or other viral pathogens. Some of the therapeutics currently under evaluation, such as GS-5734 with activity against both EBOV and MARV and BCX4430 with activity against multiple RNA viruses, are promising candidates [11]. Importantly, these drugs have demonstrated adequate triphosphate exposure at safe doses and satisfy the fundamental principles of PK/PD relationships. Further investigation utilizing well-designed and statistically powered clinical studies should be considered. However, in the absence of a current outbreak, alternative strategies including licensure through the Animal Rule may be necessary.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kuhn JH, Becker S, Ebihara H, Geisbert TW, Johnson KM, Kawaoka Y, et al. Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. Arch Virol. 2010;155:2083–103.
Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–6.
Falasca L, Agrati C, Petrosillo N, Di Caro A, Capobianchi MR, Ippolito G, et al. Molecular mechanisms of Ebola virus pathogenesis: focus on cell death. Cell Death Differ. 2015;22:1250–9.
• Misasi J, Sullivan NJ. Camouflage and misdirection: the full-on assault of ebola virus disease. Cell. 2014;159:477–86. This article highlights some of the tactics used by EBOV for immune evasion, which may serve as potential targets for therapeutic action
Bixler SL, Goff AJ. The role of cytokines and chemokines in filovirus infection. Viruses. 2015;7:5489–507.
Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011;377:849–62.
Paessler S, Walker DH. Pathogenesis of the viral hemorrhagic fevers. Annu Rev Pathol. 2013;8:411–40.
• Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011;204(Suppl 3):S810–6. This paper provides an overview of the clinical manifestations of EVD
Morgan P, Van Der Graaf PH, Arrowsmith J, Feltner DE, Drummond KS, Wegner CD, et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov Today. 2012;17:419–24.
Martins KA, Jahrling PB, Bavari S, Kuhn JH. Ebola virus disease candidate vaccines under evaluation in clinical trials. Expert Rev Vaccines. 2016;15:1101–12.
Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, Van Tongeren SA, et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508:402–5.
Taylor R, Kotian P, Warren T, Panchal R, Bavari S, Julander J, et al. BCX4430—a broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J Infect Public Health. 2016;9:220–6.
•• Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–5. This article describes the efficacy of GS-5735 when administered to nonhuman primates three days post-EBOV exposure
Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir Res. 2013;100:446–54.
Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002;46:977–81.
Spurgers KB, Sharkey CM, Warfield KL, Bavari S. Oligonucleotide antiviral therapeutics: antisense and RNA interference for highly pathogenic RNA viruses. Antivir Res. 2008;78:26–36.
Davidson E, Bryan C, Fong RH, Barnes T, Pfaff JM, Mabila M, et al. Mechanism of binding to Ebola virus glycoprotein by the ZMapp, ZMAb, and MB-003 cocktail antibodies. J Virol. 2015;89:10982–92.
Michelow IC, Lear C, Scully C, Prugar LI, Longley CB, Yantosca LM, et al. High-dose mannose-binding lectin therapy for Ebola virus infection. J Infect Dis. 2011;203:175–9.
Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. 2005;308:1643–5.
Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion R Jr, Nunneley JW, et al. Protease inhibitors targeting coronavirus and filovirus entry. Antivir Res. 2015;116:76–84.
Basu A, Mills DM, Mitchell D, Ndungo E, Williams JD, Herbert AS, et al. Novel small molecule entry inhibitors of Ebola virus. J Infect Dis. 2015;212(Suppl 2):S425–34.
Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, et al. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc Natl Acad Sci U S A. 2011;108:8426–31.
Qiu X, Wong G, Fernando L, Audet J, Bello A, Strong J, et al. mAbs and Ad-vectored IFN-alpha therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci Transl Med. 2013;5:207ra143.
Smith LM, Hensley LE, Geisbert TW, Johnson J, Stossel A, Honko A, et al. Interferon-beta therapy prolongs survival in rhesus macaque models of Ebola and Marburg hemorrhagic fever. J Infect Dis. 2013;208:310–8.
Geisbert TW, Hensley LE, Jahrling PB, Larsen T, Geisbert JB, Paragas J, et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet. 2003;362:1953–8.
Hensley LE, Stevens EL, Yan SB, Geisbert JB, Macias WL, Larsen T, et al. Recombinant human activated protein C for the postexposure treatment of Ebola hemorrhagic fever. J Infect Dis. 2007;196(Suppl 2):S390–9.
Wolf T, Kann G, Becker S, Stephan C, Brodt HR, de Leuw P, et al. Severe Ebola virus disease with vascular leakage and multiorgan failure: treatment of a patient in intensive care. Lancet. 2015;385:1428–35.
BioCryst Pharmaceuticals I. BioCryst announces study results for BCX4430 in a non-human primate model of Ebola virus infection. 2014.
BioCryst Pharmaceuticals I. BioCryst announces positive study results for BCX4430 delayed treatment of Ebola virus infection in a non-human primate model. 2016.
Oestereich L, Ludtke A, Wurr S, Rieger T, Munoz-Fontela C, Gunther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antivir Res. 2014;105:17–21.
Smither SJ, Eastaugh LS, Steward JA, Nelson M, Lenk RP, Lever MS. Post-exposure efficacy of oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model. Antivir Res. 2014;104:153–5.
• Salata C, Calistri A, Parolin C, Baritussio A, Palu G. Antiviral activity of cationic amphiphilic drugs. Expert Rev Anti-Infect Ther. 2017;15:483–92. This article describes the use of CADs in drug repurposing efforts
• Madrid PB, Chopra S, Manger ID, Gilfillan L, Keepers TR, Shurtleff AC, et al. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS One. 2013;8:e60579. This paper describes in vitro screening efforts aimed at identifying potential drugs for repurposing efforts.
Kouznetsova J, Sun W, Martinez-Romero C, Tawa G, Shinn P, Chen CZ, et al. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg Microbes Infect. 2014;3:e84.
Madrid PB, Panchal RG, Warren TK, Shurtleff AC, Endsley AN, Green CE, et al. Evaluation of Ebola virus inhibitors for drug repurposing. ACS Infect Dis. 2015;1:317–26.
Ruscoe JE, Jewell H, Maggs JL, O'Neill PM, Storr RC, Ward SA, et al. The effect of chemical substitution on the metabolic activation, metabolic detoxication, and pharmacological activity of amodiaquine in the mouse. J Pharmacol Exp Ther. 1995;273:393–404.
Falzarano D, Safronetz D, Prescott J, Marzi A, Feldmann F, Feldmann H. Lack of protection against ebola virus from chloroquine in mice and hamsters. Emerg Infect Dis. 2015;21:1065–7.
Dowall SD, Bosworth A, Watson R, Bewley K, Taylor I, Rayner E, et al. Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig model. J Gen Virol. 2015;96:3484–92.
Mzayek F, Deng H, Mather FJ, Wasilevich EC, Liu H, Hadi CM, et al. Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers. PLoS Clin Trials. 2007;2:e6.
Walker O, Birkett DJ, Alvan G, Gustafsson LL, Sjoqvist F. Characterization of chloroquine plasma protein binding in man. Br J Clin Pharmacol. 1983;15:375–7.
Johansen LM, Brannan JM, Delos SE, Shoemaker CJ, Stossel A, Lear C, et al. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med. 2013;5:190ra79.
Ghobadi C, Mirhosseini N, Shiran MR, Moghadamnia A, Lennard MS, Ledger WL, et al. Single-dose pharmacokinetic study of clomiphene citrate isomers in anovular patients with polycystic ovary disease. J Clin Pharmacol. 2009;49:147–54.
O'Regan RM, Cisneros A, England GM, MacGregor JI, Muenzner HD, Assikis VJ, et al. Effects of the antiestrogens tamoxifen, toremifene, and ICI 182,780 on endometrial cancer growth. J Natl Cancer Inst. 1998;90:1552–8.
Taras TL, Wurz GT, Linares GR, DeGregorio MW. Clinical pharmacokinetics of toremifene. Clin Pharmacokinet. 2000;39:327–34.
Salata C, Baritussio A, Munegato D, Calistri A, Ha HR, Bigler L, et al. Amiodarone and metabolite MDEA inhibit Ebola virus infection by interfering with the viral entry process. Pathog Dis. 2015;73
Gehring G, Rohrmann K, Atenchong N, Mittler E, Becker S, Dahlmann F, et al. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J Antimicrob Chemother. 2014;69:2123–31.
Greenberg ML, Lerman BB, Shipe JR, Kaiser DL, DiMarco JP. Relation between amiodarone and desethylamiodarone plasma concentrations and electrophysiologic effects, efficacy and toxicity. J Am Coll Cardiol. 1987;9:1148–55.
Wang F, Agnihotri A, Adabag S, Roukoz H, Krueger K, Tholakanahalli V, et al. Clinical utility of plasma amiodarone level measurement: a single center experience. JICRM. 2011;2:508–11.
Girard AE, Girard D, English AR, Gootz TD, Cimochowski CR, Faiella JA, et al. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987;31:1948–54.
Johansen LM, DeWald LE, Shoemaker CJ, Hoffstrom BG, Lear-Rooney CM, Stossel A, et al. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci Transl Med. 2015;5:290ra89.
Wang JS, DeVane CL, Gibson BB, Donovan JL, Markowitz JS, Zhu HJ. Population pharmacokinetic analysis of drug-drug interactions among risperidone, bupropion, and sertraline in CF1 mice. Psychopharmacology. 2006;183:490–9.
WHO. Categorization and prioritization of drugs for consideration for testing or use in patients infected with Ebola. 2015.
• Haque A, Hober D, Blondiaux J. Addressing therapeutic options for Ebola virus infection in current and future outbreaks. Antimicrob Agents Chemother. 2015;59:5892–902. This article summarizes the current state of therapeutics for treatment of EVD
McCarthy SD, Majchrzak-Kita B, Racine T, Kozlowski HN, Baker DP, Hoenen T, et al. A rapid screening assay identifies monotherapy with interferon-β and combination therapies with nucleoside analogs as effective inhibitors of Ebola virus. PLoS Negl Trop Dis. 2016;10:e0004364.
Jahrling PB, Geisbert TW, Geisbert JB, Swearengen JR, Bray M, Jaax NK, et al. Evaluation of immune globulin and recombinant interferon-alpha2b for treatment of experimental Ebola virus infections. J Infect Dis. 1999;179(Suppl 1):S224–34.
•• Geisbert TW, Lee AC, Robbins M, Geisbert JB, Honko AN, Sood V, et al. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet. 2010;375:1896–905. This paper describes the ability of siRNA therapy to protect nonhuman primates from EBOV infection
Thi EP, Mire CE, Lee AC, Geisbert JB, Zhou JZ, Agans KN, et al. Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates. Nature. 2015;521:362–5.
Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53.
Jahrling PB, Geisbert JB, Swearengen JR, Larsen T, Geisbert TW. Ebola hemorrhagic fever: evaluation of passive immunotherapy in nonhuman primates. J Infect Dis. 2007;196(Suppl 2):S400–3.
Dye JM, Herbert AS, Kuehne AI, Barth JF, Muhammad MA, Zak SE, et al. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci U S A. 2012;109:5034–9.
Iversen PL, Warren TK, Wells JB, Garza NL, Mourich DV, Welch LS, et al. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections. Viruses. 2012;4:2806–30.
Warren TK, Warfield KL, Wells J, Swenson DL, Donner KS, Van Tongeren SA, et al. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nat Med. 2010;16:991–4.
Warren TK, Whitehouse CA, Wells J, Welch L, Heald AE, Charleston JS, et al. A single phosphorodiamidate morpholino oligomer targeting VP24 protects rhesus monkeys against lethal Ebola virus infection. MBio 2015;6.
Zhao Z, Martin C, Fan R, Bourne PE, Xie L. Drug repurposing to target Ebola virus replication and virulence using structural systems pharmacology. BMC Bioinformatics. 2016;17:90.
Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, Geisbert JB, et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am J Pathol. 2003;163:2347–70.
Geisbert TW, Young HA, Jahrling PB, Davis KJ, Larsen T, Kagan E, et al. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am J Pathol. 2003;163:2371–82.
CDC. Ebola Survivors Questions and Answers. 2016.
Vallee E, Azoulay-Dupuis E, Pocidalo JJ, Bergogne-Berezin E. Activity and local delivery of azithromycin in a mouse model of Haemophilus influenzae lung infection. Antimicrob Agents Chemother. 1992;36:1412–7.
Enterlein S, Warfield KL, Swenson DL, Stein DA, Smith JL, Gamble CS, et al. VP35 knockdown inhibits Ebola virus amplification and protects against lethal infection in mice. Antimicrob Agents Chemother. 2006;50:984–93.
Bente D, Gren J, Strong JE, Feldmann H. Disease modeling for Ebola and Marburg viruses. Dis Model Mech. 2009;2:12–7.
Lanier R, Trost L, Tippin T, Lampert B, Robertson A, Foster S, et al. Development of CMX001 for the treatment of poxvirus infections. Viruses. 2010;2:2740–62.
Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–12.
Dunning J, Kennedy SB, Antierens A, Whitehead J, Ciglenecki I, Carson G, et al. Experimental treatment of Ebola virus disease with brincidofovir. PLoS One. 2016;11:e0162199.
Florescu DF, Kalil AC, Hewlett AL, Schuh AJ, Stroher U, Uyeki TM, et al. Administration of brincidofovir and convalescent plasma in a patient with Ebola virus disease. Clin Infect Dis. 2015;61:969–73.
Dornemann J, Burzio C, Ronsse A, Sprecher A, De Clerck H, Van Herp M, et al. First newborn baby to receive experimental therapies survives Ebola virus disease. J Infect Dis. 2017;
Kraft CS, Hewlett AL, Koepsell S, Winkler AM, Kratochvil CJ, Larson L, et al. The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clin Infect Dis. 2015;61:496–502.
Dunning J, Sahr F, Rojek A, Gannon F, Carson G, Idriss B, et al. Experimental treatment of Ebola virus disease with TKM-130803: a single-arm phase 2 clinical trial. PLoS Med. 2016;13:e1001997.
Sissoko D, Laouenan C, Folkesson E, M'Lebing AB, Beavogui AH, Baize S, et al. Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled. Single-Arm Proof-of-Concept Trial in Guinea PLoS Med. 2016;13:e1001967.
Schibler M, Vetter P, Cherpillod P, Petty TJ, Cordey S, Vieille G, et al. Clinical features and viral kinetics in a rapidly cured patient with Ebola virus disease: a case report. Lancet Infect Dis. 2015;15:1034–40.
Mora-Rillo M, Arsuaga M, Ramirez-Olivencia G, de la Calle F, Borobia AM, Sanchez-Seco P, et al. Acute respiratory distress syndrome after convalescent plasma use: treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir Med. 2015;3:554–62.
Bai CQ, Mu JS, Kargbo D, Song YB, Niu WK, Nie WM, et al. Clinical and virological characteristics of Ebola virus disease patients treated with favipiravir (T-705)—Sierra Leone, 2014. Clin Infect Dis. 2016;63:1288–94.
•• Group PIW, Multi-National PIIST, Davey RT Jr, Dodd L, Proschan MA, Neaton J, et al. A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med. 2016;375:1448–56. This article highlights the feasibility of conducting a large-scale clinical trial in an outbreak setting
van Griensven J, Edwards T, de Lamballerie X, Semple MG, Gallian P, Baize S, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374:33–42.
Mupapa K, Massamba M, Kibadi K, Kuvula K, Bwaka A, Kipasa M, et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis. 1999;179(Suppl 1):S18–23.
Gao J, Yin L. Drug development for controlling Ebola epidemic—a race against time. Drug Discov Ther. 2014;8:229–31.
Florescu DF, Keck MA. Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev Anti-Infect Ther. 2014;12:1171–8.
Pettit DK, Rogers RS, Arthur K, Brodsky Y, Clark RH, Crowell C, et al. CHO cell production and sequence improvement in the 13C6FR1 anti-Ebola antibody. MAbs. 2016;8:347–57.
Deen GF, Knust B, Broutet N, Sesay FR, Formenty P, Ross C, et al. Ebola RNA persistence in semen of Ebola virus disease survivors—preliminary report. N Engl J Med. 2015;
Uyeki TM, Erickson BR, Brown S, McElroy AK, Cannon D, Gibbons A, et al. Ebola virus persistence in semen of male survivors. Clin Infect Dis. 2016;62:1552–5.
Christie A, Davies-Wayne GJ, Cordier-Lassalle T, Blackley DJ, Laney AS, Williams DE, et al. Possible sexual transmission of Ebola virus—Liberia, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:479–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sandra L. Bixler, Allen J. Duplantier, and Sina Bavari declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Viral Infections
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bixler, S.L., Duplantier, A.J. & Bavari, S. Discovering Drugs for the Treatment of Ebola Virus. Curr Treat Options Infect Dis 9, 299–317 (2017). https://doi.org/10.1007/s40506-017-0130-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40506-017-0130-z