Abstract
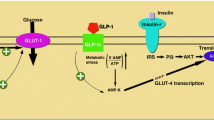
The glucagon-like peptide-1 (GLP-1) axis has emerged as a major therapeutic target for the treatment of type 2 diabetes and, recently, of obesity. The insulinotropic activity of the native incretin hormone GLP-1(7–36)amide, which is mainly exerted through a unique G protein-coupled receptor (GLP-1R), is terminated via enzymatic cleavage by dipeptidyl peptidase-IV that generates a C-terminal GLP-1 metabolite GLP-1(9–36)amide, the major circulating form in plasma. GLP-1(28–36)amide and GLP-1(32–36)amide are further cleavage products derived from GLP-1(7–36)amide and GLP-1(9–36)amide by the action of a neutral endopeptidase known as neprilysin. Until recently, GLP-1-derived metabolites were generally considered metabolically inactive. However, emerging evidence indicates that GLP-1 byproducts have insulinomimetic activities that may contribute to the pleiotropic effects of GLP-1 independently of the canonical GLP-1R. The recent studies reporting the beneficial effects of the administration of these metabolites in vivo and in vitro are the focus of this review. Collectively, these results suggest that GLP-1 metabolites inhibit hepatic glucose production, exert antioxidant cardio- and neuroprotective actions, reduce oxidative stress in vasculature and have both anti-apoptotic and proliferative effects in pancreatic β-cells, putatively by the modulation of mitochondrial functions. These findings have implication in energy homeostasis, obesity and its associated metabolic and cardiovascular complications as well as incretin-based therapies for the treatment of diabetes and obesity.


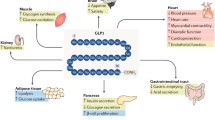
Modified from [26]
Similar content being viewed by others
References
Kieffer TJ, Habener JF (1999) The glucagon-like peptides. Endocr Rev 20(6):876–913. doi:10.1210/edrv.20.6.0385
Qualmann C, Nauck MA, Holst JJ, Orskov C, Creutzfeldt W (1995) Insulinotropic actions of intravenous glucagon-like peptide-1 (GLP-1) [7–36 amide] in the fasting state in healthy subjects. Acta Diabetol 32(1):13–16
Fehmann HC, Habener JF (1992) Insulinotropic hormone glucagon-like peptide-I(7–37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology 130(1):159–166. doi:10.1210/endo.130.1.1309325
Wang Y, Perfetti R, Greig NH, Holloway HW, DeOre KA, Montrose-Rafizadeh C, Elahi D, Egan JM (1997) Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J Clin Invest 99(12):2883–2889. doi:10.1172/JCI119482
Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA (1996) Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7–36) amide in type I diabetic patients. Diabetes Care 19(6):580–586
Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R (2003) Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 144(12):5149–5158. doi:10.1210/en.2003-0323
Perfetti R, Zhou J, Doyle ME, Egan JM (2000) Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 141(12):4600–4605. doi:10.1210/endo.141.12.7806
Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ (1993) Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci 38(4):665–673
Flint A, Raben A, Astrup A, Holst JJ (1998) Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 101(3):515–520. doi:10.1172/JCI990
Baggio LL, Drucker DJ (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132(6):2131–2157. doi:10.1053/j.gastro.2007.03.054
Thorens B (1992) Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA 89(18):8641–8645
Wheeler MB, Lu M, Dillon JS, Leng XH, Chen C, Boyd AE 3rd (1993) Functional expression of the rat glucagon-like peptide-I receptor, evidence for coupling to both adenylyl cyclase and phospholipase-C. Endocrinology 133(1):57–62. doi:10.1210/endo.133.1.8391428
Montrose-Rafizadeh C, Avdonin P, Garant MJ, Rodgers BD, Kole S, Yang H, Levine MA, Schwindinger W, Bernier M (1999) Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology 140(3):1132–1140. doi:10.1210/endo.140.3.6550
Guglielmi V, Maresca L, D’Adamo M, Di Roma M, Lanzillo C, Federici M, Lauro D, Preziosi P, Bellia A, Sbraccia P (2014) Age-related different relationships between ectopic adipose tissues and measures of central obesity in sedentary subjects. PLoS One 9(7):e103381. doi:10.1371/journal.pone.0103381
Guglielmi V, Cardellini M, Cinti F, Corgosinho F, Cardolini I, D’Adamo M, Zingaretti MC, Bellia A, Lauro D, Gentileschi P, Federici M, Cinti S, Sbraccia P (2015) Omental adipose tissue fibrosis and insulin resistance in severe obesity. Nutr Diabetes 5:e175. doi:10.1038/nutd.2015.22
Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, Amour J, Leprince P, Dutour A, Clement K, Hatem SN (2015) Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J 36(13):795–805a. doi:10.1093/eurheartj/eht099
Guglielmi V, D’Adamo M, Bellia A, Ciotto RT, Federici M, Lauro D, Sbraccia P (2015) Iron status in obesity: an independent association with metabolic parameters and effect of weight loss. Nutr Metab Cardiovasc Dis 25(6):541–547. doi:10.1016/j.numecd.2015.02.012
Lovshin JA, Drucker DJ (2009) Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5(5):262–269. doi:10.1038/nrendo.2009.48
Amori RE, Lau J, Pittas AG (2007) Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 298(2):194–206. doi:10.1001/jama.298.2.194
Boland CL, Degeeter M, Nuzum DS, Tzefos M (2013) Evaluating second-line treatment options for type 2 diabetes: focus on secondary effects of GLP-1 agonists and DPP-4 inhibitors. Ann Pharmacother 47(4):490–505. doi:10.1345/aph.1R444
Nauck M (2016) Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab 18(3):203–216. doi:10.1111/dom.12591
Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rossner S, Savolainen MJ, Van Gaal L (2012) Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 36(6):843–854. doi:10.1038/ijo.2011.158
Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME (2009) Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374(9701):1606–1616. doi:10.1016/S0140-6736(09)61375-1
Lombardo M, Bellia A, Padua E, Annino G, Guglielmi V, D’Adamo M, Iellamo F, Sbraccia P (2014) Morning meal more efficient for fat loss in a 3-month lifestyle intervention. J Am Coll Nutr 33(3):198–205. doi:10.1080/07315724.2013.863169
Bellia A, Salli M, Lombardo M, D’Adamo M, Guglielmi V, Tirabasso C, Giordani L, Federici M, Lauro D, Foti C, Sbraccia P (2014) Effects of whole body vibration plus diet on insulin-resistance in middle-aged obese subjects. Int J Sports Med 35(6):511–516. doi:10.1055/s-0033-1354358
Tomas E, Habener JF (2010) Insulin-like actions of glucagon-like peptide-1: a dual receptor hypothesis. Trends Endocrinol Metab 21(2):59–67. doi:10.1016/j.tem.2009.11.007
Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ (1994) Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 43(4):535–539
Hansen L, Deacon CF, Orskov C, Holst JJ (1999) Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 140(11):5356–5363. doi:10.1210/endo.140.11.7143
Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ (1995) Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 44(9):1126–1131
Egan JM, Meneilly GS, Habener JF, Elahi D (2002) Glucagon-like peptide-1 augments insulin-mediated glucose uptake in the obese state. J Clin Endocrinol Metab 87(8):3768–3773. doi:10.1210/jcem.87.8.8743
Plamboeck A, Holst JJ, Carr RD, Deacon CF (2005) Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia 48(9):1882–1890. doi:10.1007/s00125-005-1847-7
Sharma R, McDonald TS, Eng H, Limberakis C, Stevens BD, Patel S, Kalgutkar AS (2013) In vitro metabolism of the glucagon-like peptide-1 (GLP-1)-derived metabolites GLP-1(9–36)amide and GLP-1(28–36)amide in mouse and human hepatocytes. Drug Metab Dispos 41(12):2148–2157. doi:10.1124/dmd.113.054254
Nikolaidis LA, Elahi D, Shen YT, Shannon RP (2005) Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 289(6):H2401–H2408. doi:10.1152/ajpheart.00347.2005
Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M (2008) Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117(18):2340–2350. doi:10.1161/CIRCULATIONAHA.107.739938
Ban K, Kim KH, Cho CK, Sauve M, Diamandis EP, Backx PH, Drucker DJ, Husain M (2010) Glucagon-like peptide (GLP)-1(9–36)amide-mediated cytoprotection is blocked by exendin(9–39) yet does not require the known GLP-1 receptor. Endocrinology 151(4):1520–1531. doi:10.1210/en.2009-1197
Ossum A, van Deurs U, Engstrom T, Jensen JS, Treiman M (2009) The cardioprotective and inotropic components of the postconditioning effects of GLP-1 and GLP-1(9–36)a in an isolated rat heart. Pharmacol Res 60(5):411–417. doi:10.1016/j.phrs.2009.06.004
Sonne DP, Engstrom T, Treiman M (2008) Protective effects of GLP-1 analogues exendin-4 and GLP-1(9–36) amide against ischemia-reperfusion injury in rat heart. Regul Pept 146(1–3):243–249. doi:10.1016/j.regpep.2007.10.001
Ussher JR, Baggio LL, Campbell JE, Mulvihill EE, Kim M, Kabir MG, Cao X, Baranek BM, Stoffers DA, Seeley RJ, Drucker DJ (2014) Inactivation of the cardiomyocyte glucagon-like peptide-1 receptor (GLP-1R) unmasks cardiomyocyte-independent GLP-1R-mediated cardioprotection. Mol Metab 3(5):507–517. doi:10.1016/j.molmet.2014.04.009
Robinson E, Tate M, Lockhart S, McPeake C, O’Neill KM, Edgar KS, Calderwood D, Green BD, McDermott BJ, Grieve DJ (2016) Metabolically-inactive glucagon-like peptide-1(9–36)amide confers selective protective actions against post-myocardial infarction remodelling. Cardiovasc Diabetol 15:65. doi:10.1186/s12933-016-0386-5
Frangogiannis NG (2015) Inflammation in cardiac injury, repair and regeneration. Curr Opin Cardiol 30(3):240–245. doi:10.1097/HCO.0000000000000158
Porter KE, Turner NA (2009) Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther 123(2):255–278. doi:10.1016/j.pharmthera.2009.05.002
Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ (2008) GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys 478(2):136–142. doi:10.1016/j.abb.2008.08.001
Giacco F, Du X, Carratu A, Gerfen GJ, D’Apolito M, Giardino I, Rasola A, Marin O, Divakaruni AS, Murphy AN, Shah MS, Brownlee M (2015) GLP-1 cleavage product reverses persistent ROS generation after transient hyperglycemia by disrupting an ROS-generating feedback loop. Diabetes 64(9):3273–3284. doi:10.2337/db15-0084db15-0084
Hvidberg A, Nielsen MT, Hilsted J, Orskov C, Holst JJ (1994) Effect of glucagon-like peptide-1 (proglucagon 78–107amide) on hepatic glucose production in healthy man. Metabolism 43(1):104–108
Lee YS, Shin S, Shigihara T, Hahm E, Liu MJ, Han J, Yoon JW, Jun HS (2007) Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes 56(6):1671–1679. doi:10.2337/db06-1182
Prigeon RL, Quddusi S, Paty B, D’Alessio DA (2003) Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab 285(4):E701–E707. doi:10.1152/ajpendo.00024.2003
Seghieri M, Rebelos E, Gastaldelli A, Astiarraga BD, Casolaro A, Barsotti E, Pocai A, Nauck M, Muscelli E, Ferrannini E (2013) Direct effect of GLP-1 infusion on endogenous glucose production in humans. Diabetologia 56(1):156–161. doi:10.1007/s00125-012-2738-3
Meneilly GS, McIntosh CH, Pederson RA, Habener JF, Gingerich R, Egan JM, Finegood DT, Elahi D (2001) Effect of glucagon-like peptide 1 on non-insulin-mediated glucose uptake in the elderly patient with diabetes. Diabetes Care 24(11):1951–1956
Shalev A, Ninnis R, Keller U (1998) Effects of glucagon-like peptide 1 (7–36 amide) on glucose kinetics during somatostatin-induced suppression of insulin secretion in healthy men. Horm Res 49(5):221–225
Meier JJ, Gethmann A, Nauck MA, Gotze O, Schmitz F, Deacon CF, Gallwitz B, Schmidt WE, Holst JJ (2006) The glucagon-like peptide-1 metabolite GLP-1-(9–36) amide reduces postprandial glycemia independently of gastric emptying and insulin secretion in humans. Am J Physiol Endocrinol Metab 290(6):E1118–E1123. doi:10.1152/ajpendo.00576.2005
Elahi D, Egan JM, Shannon RP, Meneilly GS, Khatri A, Habener JF, Andersen DK (2008) GLP-1 (9–36) amide, cleavage product of GLP-1 (7–36) amide, is a glucoregulatory peptide. Obesity (Silver Spring) 16(7):1501–1509. doi:10.1038/oby.2008.229
Tomas E, Stanojevic V, Habener JF (2010) GLP-1 (9–36) amide metabolite suppression of glucose production in isolated mouse hepatocytes. Horm Metab Res 42(9):657–662. doi:10.1055/s-0030-1253421
Ip W, Shao W, Chiang YT, Jin T (2013) GLP-1-derived nonapeptide GLP-1(28–36)amide represses hepatic gluconeogenic gene expression and improves pyruvate tolerance in high-fat diet-fed mice. Am J Physiol Endocrinol Metab 305(11):E1348–E1358. doi:10.1152/ajpendo.00376.2013
Tomas E, Stanojevic V, Habener JF (2011) GLP-1-derived nonapeptide GLP-1(28–36)amide targets to mitochondria and suppresses glucose production and oxidative stress in isolated mouse hepatocytes. Regul Pept 167(2–3):177–184. doi:10.1016/j.regpep.2011.01.003
Elahi D, Angeli FS, Vakilipour A, Carlson OD, Tomas E, Egan JM, Habener JF, Shannon RP (2014) GLP-1(32–36)amide, a novel pentapeptide cleavage product of GLP-1, modulates whole body glucose metabolism in dogs. Peptides 59:20–24. doi:10.1016/j.peptides.2014.06.004
Panjwani N, Mulvihill EE, Longuet C, Yusta B, Campbell JE, Brown TJ, Streutker C, Holland D, Cao X, Baggio LL, Drucker DJ (2013) GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(−/−) mice. Endocrinology 154(1):127–139. doi:10.1210/en.2012-1937
Aviv V, Meivar-Levy I, Rachmut IH, Rubinek T, Mor E, Ferber S (2009) Exendin-4 promotes liver cell proliferation and enhances the PDX-1-induced liver to pancreas transdifferentiation process. J Biol Chem 284(48):33509–33520. doi:10.1074/jbc.M109.017608
Bullock BP, Heller RS, Habener JF (1996) Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 137(7):2968–2978. doi:10.1210/endo.137.7.8770921
Korner M, Stockli M, Waser B, Reubi JC (2007) GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 48(5):736–743. doi:10.2967/jnumed.106.038679
Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, Anania FA (2010) Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 51(5):1584–1592. doi:10.1002/hep.23569
Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, Faraci G, Pacetti D, Vivarelli M, Nicolini D, Garelli P, Casini A, Manco M, Mingrone G, Risaliti A, Frega GN, Benedetti A, Gastaldelli A (2011) Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int 31(9):1285–1297. doi:10.1111/j.1478-3231.2011.02462.x
Burcelin R, Da Costa A, Drucker D, Thorens B (2001) Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes 50(8):1720–1728
Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A (1996) Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol 271(5 Pt 1):E808–E813
Johnson KM, Edgerton DS, Rodewald T, Scott M, Farmer B, Neal D, Cherrington AD (2007) Intraportal GLP-1 infusion increases nonhepatic glucose utilization without changing pancreatic hormone levels. Am J Physiol Endocrinol Metab 293(4):E1085–E1091. doi:10.1152/ajpendo.00275.2007
Burmeister MA, Ferre T, Ayala JE, King EM, Holt RM (2012) Acute activation of central GLP-1 receptors enhances hepatic insulin action and insulin secretion in high-fat-fed, insulin resistant mice. Am J Physiol Endocrinol Metab 302(3):E334–E343. doi:10.1152/ajpendo.00409.2011
Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ (2008) Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 57(8):2046–2054. doi:10.2337/db07-1824
Tomas E, Wood JA, Stanojevic V, Habener JF (2011) Glucagon-like peptide-1(9–36)amide metabolite inhibits weight gain and attenuates diabetes and hepatic steatosis in diet-induced obese mice. Diabetes Obes Metab 13(1):26–33. doi:10.1111/j.1463-1326.2010.01316.x
Tomas E, Wood JA, Stanojevic V, Habener JF (2011) GLP-1-derived nonapeptide GLP-1(28–36)amide inhibits weight gain and attenuates diabetes and hepatic steatosis in diet-induced obese mice. Regul Pept 169(1–3):43–48. doi:10.1016/j.regpep.2011.04.006
Tomas E, Stanojevic V, McManus K, Khatri A, Everill P, Bachovchin WW, Habener JF (2015) GLP-1(32–36)amide pentapeptide increases basal energy expenditure and inhibits weight gain in obese mice. Diabetes 64(7):2409–2419. doi:10.2337/db14-1708
Lefebvre P, Chinetti G, Fruchart JC, Staels B (2006) Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest 116(3):571–580. doi:10.1172/JCI27989
Liu Z, Stanojevic V, Brindamour LJ, Habener JF (2012) GLP1-derived nonapeptide GLP1(28–36)amide protects pancreatic beta-cells from glucolipotoxicity. J Endocrinol 213(2):143–154. doi:10.1530/JOE-11-0328
Shao W, Wang Z, Ip W, Chiang YT, Xiong X, Chai T, Xu C, Wang Q, Jin T (2013) GLP-1(28–36) improves beta-cell mass and glucose disposal in streptozotocin-induced diabetic mice and activates cAMP/PKA/beta-catenin signaling in beta-cells in vitro. Am J Physiol Endocrinol Metab 304(12):E1263–E1272. doi:10.1152/ajpendo.00600.2012
Zraika S, Hull RL, Udayasankar J, Clark A, Utzschneider KM, Tong J, Gerchman F, Kahn SE (2007) Identification of the amyloid-degrading enzyme neprilysin in mouse islets and potential role in islet amyloidogenesis. Diabetes 56(2):304–310. doi:10.2337/db06-0430
Holscher C (2012) Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection. CNS Drugs 26(10):871–882. doi:10.2165/11635890-000000000-00000
Holscher C (2014) Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol 221(1):T31–T41. doi:10.1530/JOE-13-0221
Ma T, Du X, Pick JE, Sui G, Brownlee M, Klann E (2012) Glucagon-like peptide-1 cleavage product GLP-1(9–36) amide rescues synaptic plasticity and memory deficits in Alzheimer’s disease model mice. J Neurosci 32(40):13701–13708. doi:10.1523/JNEUROSCI.2107-12.2012
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795. doi:10.1038/nature05292
Massaad CA, Klann E (2011) Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal 14(10):2013–2054. doi:10.1089/ars.2010.3208
Hooper C, Killick R, Lovestone S (2008) The GSK3 hypothesis of Alzheimer’s disease. J Neurochem 104(6):1433–1439. doi:10.1111/j.1471-4159.2007.05194.x
Beinborn M, Worrall CI, McBride EW, Kopin AS (2005) A human glucagon-like peptide-1 receptor polymorphism results in reduced agonist responsiveness. Regul Pept 130(1–2):1–6. doi:10.1016/j.regpep.2005.05.001
Demers A, McNicoll N, Febbraio M, Servant M, Marleau S, Silverstein R, Ong H (2004) Identification of the growth hormone-releasing peptide binding site in CD36: a photoaffinity cross-linking study. Biochem J 382(Pt 2):417–424. doi:10.1042/BJ20040036
Szeto HH (2006) Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J 8(2):E277–E283. doi:10.1208/aapsj080232
Zhao KH, Zhang J, Tu JM, Bohm S, Ploscher M, Eichacker L, Bubenzer C, Scheer H, Wang X, Zhou M (2007) Lyase activities of CpcS- and CpcT-like proteins from Nostoc PCC7120 and sequential reconstitution of binding sites of phycoerythrocyanin and phycocyanin beta-subunits. J Biol Chem 282(47):34093–34103. doi:10.1074/jbc.M703038200
Brun C, Philip-Couderc P, Raggenbass M, Roatti A, Baertschi AJ (2006) Intracellular targeting of truncated secretory peptides in the mammalian heart and brain. FASEB J 20(6):732–734. doi:10.1096/fj.05-4338fje
Lemire BD, Fankhauser C, Baker A, Schatz G (1989) The mitochondrial targeting function of randomly generated peptide sequences correlates with predicted helical amphiphilicity. J Biol Chem 264(34):20206–20215
Yamada H, Chounan R, Higashi Y, Kurihara N, Kido H (2004) Mitochondrial targeting sequence of the influenza A virus PB1-F2 protein and its function in mitochondria. FEBS Lett 578(3):331–336. doi:10.1016/j.febslet.2004.11.017
Chatre L, Matheson LA, Jack AS, Hanton SL, Brandizzi F (2009) Efficient mitochondrial targeting relies on co-operation of multiple protein signals in plants. J Exp Bot 60(3):741–749. doi:10.1093/jxb/ern319
Chiang YT, Ip W, Jin T (2012) The role of the Wnt signaling pathway in incretin hormone production and function. Front Physiol 3:273. doi:10.3389/fphys.2012.00273
Ryan AS, Egan JM, Habener JF, Elahi D (1998) Insulinotropic hormone glucagon-like peptide-1-(7–37) appears not to augment insulin-mediated glucose uptake in young men during euglycemia. J Clin Endocrinol Metab 83(7):2399–2404. doi:10.1210/jcem.83.7.4988
Vahl TP, Paty BW, Fuller BD, Prigeon RL, D’Alessio DA (2003) Effects of GLP-1-(7–36)NH2, GLP-1-(7-37), and GLP-1- (9–36)NH2 on intravenous glucose tolerance and glucose-induced insulin secretion in healthy humans. J Clin Endocrinol Metab 88(4):1772–1779. doi:10.1210/jc.2002-021479
Rolin B, Deacon CF, Carr RD, Ahren B (2004) The major glucagon-like peptide-1 metabolite, GLP-1-(9–36)-amide, does not affect glucose or insulin levels in mice. Eur J Pharmacol 494(2–3):283–288. doi:10.1016/j.ejphar.2004.05.013
Standeven KF, Hess K, Carter AM, Rice GI, Cordell PA, Balmforth AJ, Lu B, Scott DJ, Turner AJ, Hooper NM, Grant PJ (2011) Neprilysin, obesity and the metabolic syndrome. Int J Obes (Lond) 35(8):1031–1040. doi:10.1038/ijo.2010.227
Campbell JE, Drucker DJ (2013) Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 17(6):819–837. doi:10.1016/j.cmet.2013.04.008
Simonsen L, Pilgaard S, Carr RD, Kanstrup AB, Holst JJ, Deacon CF (2009) Inhibition of neutral endopeptidase 24.11 does not potentiate the improvement in glycemic control obtained with dipeptidyl peptidase-4 inhibition in diabetic Goto-Kakizaki rats. Horm Metab Res 41(11):851–853. doi:10.1055/s-0029-1225609
Malm-Erjefalt M, Bjornsdottir I, Vanggaard J, Helleberg H, Larsen U, Oosterhuis B, van Lier JJ, Zdravkovic M, Olsen AK (2010) Metabolism and excretion of the once-daily human glucagon-like peptide-1 analog liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase. Drug Metab Dispos 38(11):1944–1953. doi:10.1124/dmd.110.034066
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I (2013) Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 369(14):1317–1326. doi:10.1056/NEJMoa1307684
White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F (2013) Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 369(14):1327–1335. doi:10.1056/NEJMoa1305889
Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Lam H, White WB (2015) Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 385(9982):2067–2076. doi:10.1016/S0140-6736(14)62225-X
Mulvihill EE, Varin EM, Ussher JR, Campbell JE, Bang KW, Abdullah T, Baggio LL, Drucker DJ (2016) Inhibition of dipeptidyl peptidase-4 impairs ventricular function and promotes cardiac fibrosis in high fat-fed diabetic mice. Diabetes 65(3):742–754. doi:10.2337/db15-1224
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by a grant from the Ministero della Salute (Project n. 45/RF-2013-02357791).
Conflict of interest
Valeria Guglielmi declares that she has no conflict of interest. Paolo Sbraccia declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Guglielmi, V., Sbraccia, P. GLP-1 receptor independent pathways: emerging beneficial effects of GLP-1 breakdown products. Eat Weight Disord 22, 231–240 (2017). https://doi.org/10.1007/s40519-016-0352-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-016-0352-y