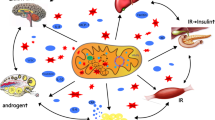
Abstract
Polycystic ovary syndrome (PCOS) is a multi-causal condition. Among the genetic causes, variations in the mitochondrial DNA (mtDNA) are increasingly recognised as causative. PCOS not only occurs in known syndromic mitochondrial disorders due to pathogenic variants in the mtDNA but also in non-syndromic mitochondrial disorders. Additionally, mtDNA variants not causing a multi-system mitochondrial disorder but exclusively PCOS have been reported. Among the syndromic mitochondrial disorders, PCOS has been described in myoclonic epilepsy with ragged-red fibre (MERRF) syndrome. Among the non-syndromic mitochondrial disorders, PCOS has been described in association with insulin resistance. Several other studies suggest that mtDNA point mutations or mtDNA deletions can be associated with PCOS without manifesting in organs other than the ovaries. Evidence from animal studies suggests that function, morphology, and biogenesis of mitochondria in ovarian tissue are generally impaired in PCOS patients. In conclusion, there is increasing evidence that mtDNA variants play a pathophysiological role in the development of PCOS. Further studies are needed to establish the causal link between mtDNA variants and PCOS.
Similar content being viewed by others
Data Availability
All data are available from the corresponding author.
Code Availability
Not applicable.
References
Joham AE, Piltonen T, Lujan ME, Kiconco S, Tay CT. Challenges in diagnosis and understanding of natural history of PCOS. Clin Endocrinol (Oxf). 2022. https://doi.org/10.1111/cen.14757.
Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to PCOS (PCOS). Hum Reprod. 2004;19(1):41–7. https://doi.org/10.1093/humrep/deh098.
Wang T, Liu Y, Lv M, Xing Q, Zhang Z, He X, Xu Y, Wei Z, Cao Y. miR-323-3p regulates the steroidogenesis and cell apoptosis in PCOS (PCOS) by targeting IGF-1. Gene. 2019;30(683):87–100. https://doi.org/10.1016/j.gene.2018.10.006.
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. PCOS. Nat Rev Dis Primers. 2016;11(2):16057. https://doi.org/10.1038/nrdp.2016.57.
Lathia T, Joshi A, Behl A, Dhingra A, Kalra B, Dua C, Bajaj K, Verma K, Malhotra N, Galagali P, Sahay R, Mittal S, Bajaj S, Moorthy S, Sharma S, Kalra S. A practitioner’s toolkit for PCOS counselling. Indian J Endocrinol Metab. 2022;26(1):17–25. https://doi.org/10.4103/ijem.ijem_411_21.
Finsterer J. Photosensitive epilepsy and PCOS as manifestations of MERRF. Case Rep Neurol Med. 2020;28(2020):8876272. https://doi.org/10.1155/2020/8876272.
Finsterer J, Zarrouk-Mahjoub S. PCOS in mitochondrial disorders due mtDNA or nDNA variants. Am J Transl Res. 2018;10(1):13–5.
Ding Y, Xia BH, Zhang CJ, Zhuo GC. Mitochondrial tRNALeu(UUR) C3275T, tRNAGln T4363C and tRNALys A8343G mutations may be associated with PCOS and metabolic syndrome. Gene. 2018;5(642):299–306. https://doi.org/10.1016/j.gene.2017.11.049.
Ye M, Hu B, Shi W, Guo F, Xu C, Li S. Mitochondrial DNA 4977 bp deletion in peripheral blood is associated with PCOS. Front Endocrinol (Lausanne). 2021;8(12): 675581. https://doi.org/10.3389/fendo.2021.675581.
Deng X, Ji D, Li X, Xu Y, Cao Y, Zou W, Liang C, Lee Marley J, Zhang Z, Wei Z, Zhou P, Liu Y, Cao Y. Polymorphisms and haplotype of mitochondrial DNA D-loop region are associated with PCOS in a Chinese population. Mitochondrion. 2021;57:173–81. https://doi.org/10.1016/j.mito.2020.12.006.
Moreno-Asso A, Altıntaş A, McIlvenna LC, Patten RK, Botella J, McAinch AJ, Rodgers RJ, Barrès R, Stepto NK. Non-cell autonomous mechanisms control mitochondrial gene dysregulation in polycystic ovary syndrome. J Mol Endocrinol. 2021;68(1):63–76. https://doi.org/10.1530/JME-21-0212.
Lerner A, Kewada D, Ahmed A, Hardy K, Christian M, Franks S. Androgen reduces mitochondrial respiration in mouse brown adipocytes: a model for disordered energy balance in polycystic ovary syndrome. Int J Mol Sci. 2020;22(1):243. https://doi.org/10.3390/ijms22010243.
Jia L, Li J, He B, Jia Y, Niu Y, Wang C, Zhao R. Abnormally activated one-carbon metabolic pathway is associated with mtDNA hypermethylation and mitochondrial malfunction in the oocytes of polycystic gilt ovaries. Sci Rep. 2016;13(6):19436. https://doi.org/10.1038/srep19436.
Yumiceba V, López-Cortés A, Pérez-Villa A, Yumiseba I, Guerrero S, García-Cárdenas JM, Armendáriz-Castillo I, Guevara-Ramírez P, Leone PE, Zambrano AK, Paz-Y-Miño C. Oncology and pharmacogenomics insights in polycystic ovary syndrome : an integrative analysis. Front Endocrinol (Lausanne). 2020;26(11):585130. https://doi.org/10.3389/fendo.2020.585130.
Kujanpää L, Arffman RK, Pesonen P, Korhonen E, Karjula S, Järvelin MR, Franks S, Tapanainen JS, Morin-Papunen L, Piltonen TT. Women with polycystic ovary syndrome are burdened with multimorbidity and medication use independent of body mass index at late fertile age: a population-based cohort study. Acta Obstet Gynecol Scand. 2022;101(7):728–36. https://doi.org/10.1111/aogs.14382.
Ali AT, Guidozzi F. Midlife women’s health consequences associated with polycystic ovary syndrome. Climacteric. 2020;23(2):116–22. https://doi.org/10.1080/13697137.2019.1679111.
Ding Y, Xia BH, Zhang CJ, Zhuo GC. Mutations in mitochondrial tRNA genes may be related to insulin resistance in women with polycystic ovary syndrome. Am J Transl Res. 2017;9(6):2984–96.
Ding Y, Zhuo G, Zhang C, Leng J. Point mutation in mitochondrial tRNA gene is associated with polycystic ovary syndrome and insulin resistance. Mol Med Rep. 2016;13(4):3169–72. https://doi.org/10.3892/mmr.2016.4916.
Wang J, Wu X. The effects of mitochondrial dysfunction on energy metabolism switch by HIF-1α signalling in granulosa cells of polycystic ovary syndrome. Endokrynol Pol. 2020;71(2):134–45. https://doi.org/10.5603/EP.a2020.0002.
Huang JC, Duan CC, Jin S, Sheng CB, Wang YS, Yue ZP, Guo B. HB-EGF induces mitochondrial dysfunction via estrogen hypersecretion in granulosa cells dependent on cAMP-PKA-JNK/ERK-Ca2+-FOXO1 pathway. Int J Biol Sci. 2022;18(5):2047–59. https://doi.org/10.7150/ijbs.69343.
Ilie IR. Advances in PCOS pathogenesis and progression-mitochondrial mutations and dysfunction. Adv Clin Chem. 2018;86:127–55. https://doi.org/10.1016/bs.acc.2018.05.003.E.
Safaei Z, Bakhshalizadeh SH, Nasr Esfahani MH, Akbari Sene A, Najafzadeh V, Soleimani M, Shirazi R. Effect of vitamin D3 on mitochondrial biogenesis in granulosa cells derived from polycystic ovary syndrome. Int J Fertil Steril. 2020;14(2):143–9. https://doi.org/10.22074/ijfs.2020.6077.
Shukla P, Mukherjee S, Patil A. Identification of variants in mitochondrial D-loop and OriL region and analysis of mitochondrial DNA copy number in women with polycystic ovary syndrome. DNA Cell Biol. 2020;39(8):1458–66. https://doi.org/10.1089/dna.2019.5323.
Reddy TV, Govatati S, Deenadayal M, Sisinthy S, Bhanoori M. Impact of mitochondrial DNA copy number and displacement loop alterations on polycystic ovary syndrome risk in south Indian women. Mitochondrion. 2019;44:35–40. https://doi.org/10.1016/j.mito.2017.12.010.
Reddy TV, Govatati S, Deenadayal M, Shivaji S, Bhanoori M. Polymorphisms in the TFAM and PGC1-α genes and their association with polycystic ovary syndrome among South Indian women. Gene. 2018;30(641):129–36. https://doi.org/10.1016/j.gene.2017.10.010.
Yang PK, Chou CH, Chang CH, Chen SU, Ho HN, Chen MJ. Changes in peripheral mitochondrial DNA copy number in metformin-treated women with polycystic ovary syndrome : a longitudinal study. Reprod Biol Endocrinol. 2020;18(1):69. https://doi.org/10.1186/s12958-020-00629-5.
Rashid N, Nigam A, Jain SK, Naqvi SH, Wajid S. Proteomic sift through serum and endometrium profiles unraveled signature proteins associated with subdued fertility and dampened endometrial receptivity in women with polycystic ovary syndrome. Cell Tissue Res. 2020;380(3):593–614. https://doi.org/10.1007/s00441-020-03171-3.
Qasemi M, Aleyasin A, Mahdian R, Ghanami Gashti N, ShabaniNashtaei M, Ashrafnezhad Z, Amidi F. Cell-free mtDNA level and its biomarker potency for ART outcome are different in follicular fluid of PCOS and non-PCOS women. Mitochondrion. 2021;59:30–6. https://doi.org/10.1016/j.mito.2021.04.003.
Lambertini L, Saul SR, Copperman AB, Hammerstad SS, Yi Z, Zhang W, Tomer Y, Kase N. Intrauterine reprogramming of the polycystic ovary syndrome : evidence from a pilot study of cord blood global methylation analysis. Front Endocrinol (Lausanne). 2017;18(8):352. https://doi.org/10.3389/fendo.2017.00352.
Ding Y, Jiang Z, Xia B, Zhang L, Zhang C, Leng J. Mitochondria-targeted antioxidant therapy for an animal model of PCOS-IR. Int J Mol Med. 2019;43(1):316–24. https://doi.org/10.3892/ijmm.2018.3977.
Song L, Yu J, Zhang D, Li X, Chen L, Cai Z, Yu C. Androgen excess induced mitochondrial abnormality in ovarian granulosa cells in a rat model of polycystic ovary syndrome. Front Endocrinol (Lausanne). 2022;17(13): 789008. https://doi.org/10.3389/fendo.2022.789008.
Chappell NR, Zhou B, Hosseinzadeh P, Schutt A, Gibbons WE, Blesson CS. Hyperandrogenemia alters mitochondrial structure and function in the oocytes of obese mouse with polycystic ovary syndrome. F S Sci. 2021;2(1):101–12. https://doi.org/10.1016/j.xfss.2020.12.001.
Chappell NR, Zhou B, Schutt AK, Gibbons WE, Blesson CS. Prenatal androgen induced lean PCOS impairs mitochondria and mRNA profiles in oocytes. Endocr Connect. 2020;9(3):261–70. https://doi.org/10.1530/EC-19-0553.
Hu M, Zhang Y, Guo X, Jia W, Liu G, Zhang J, Li J, Cui P, Sferruzzi-Perri AN, Han Y, Wu X, Ma H, Brännström M, Shao LR, Billig H. Hyperandrogenism and insulin resistance induce gravid uterine defects in association with mitochondrial dysfunction and aberrant reactive oxygen species production. Am J Physiol Endocrinol Metab. 2019;316(5):E794–809. https://doi.org/10.1152/ajpendo.00359.2018.
Yi S, Zheng B, Zhu Y, Cai Y, Sun H, Zhou J. Melatonin ameliorates excessive PINK1/Parkin-mediated mitophagy by enhancing SIRT1 expression in granulosa cells of PCOS. Am J Physiol Endocrinol Metab. 2020;319(1):E91–101. https://doi.org/10.1152/ajpendo.00006.2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Was in accordance with ethical guidelines. The study was approved by the institutional review board.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Finsterer, J. Mitochondrial Dysfunction in Polycystic Ovary Syndrome. Reprod. Sci. 30, 1435–1442 (2023). https://doi.org/10.1007/s43032-022-01100-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01100-z