Abstract
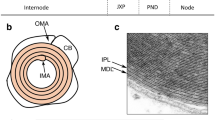
Protein zero (P0) is an integral transmembrane glycoprotein that serves as the major protein component of peripheral nerve myelin and is a member of the immunoglobulin (IgG) gene superfamily. As a cell adhesion molecule, P0 mediates homophilic adhesive interactions between Schwann cell plasma membranes and is a key structural constituent of both the major dense line and intraperiod line of compact myelin. Both the extracellular and cytoplasmic domains contribute to these interactions and evidence indicates that the post-translational modifications of the molecule, including glycosylation, acylation and phosphorylation, play an important modulatory role in adhesion and likely in the proper trafficking of P0 from the endoplasmic reticulum to the plasma membrane as well. Structural and genetic studies indicate that mutations in P0 producing human demyelinating diseases probably do so by perturbing or preventing homophilic interactions during myelination, or by producing cellular toxicity or an unstable myelin sheath. A variety of transcription factors, growth factors and neurosteroids both directly and indirectly influence P0 gene expression during maturation of the myelinating Schwann cell. Besides its structural function in myelin, P0 may have roles in the delivery of other Schwann cell proteins to their proper location, especially at or near nodes of Ranvier, and in neuronal-glial interactions.
Similar content being viewed by others
REFERENCES
Everly, J. I., Brady, R. O., and Quarles, R. H. 1973. Evidence that the major protein in rat sciatic nerve myelin is a glycoprotein. J. Neurochem. 21:329-334.
Wood, J. G. and Dawson, R. M. C. 1974. Some properties of a major structural glycoprotein of sciatic nerve. J. Neurochem. 22:627-630.
Greenfield, S., Brostoff, S., Eylar, E. H., and Morell, P. 1973. Protein composition of the peripheral nervous system. J. Neurochem. 20:1207-1216.
Kitamura, K., Suzuki, M., Susuki, A., and Uyemura, K. 1976. Purification and partial characterization of two glycoproteins in bovine peripheral nerve membrane. Biochim. Biophys. Acta 455:806-816.
Roomi, M. W., Ishaque, A., Khan, A. R., and Eylar, E. H. 1978. The P0 protein: the major glycoprotein of peripheral nerve myelin. Biochim. Biophys. Acta 536:112-121.
Ishaque, A., Roomi, M. W., Szymanski, I., Kowalski, S., and Eylar, E. H. 1980. The P0 glycoprotein of peripheral nerve myelin. Can. J. Biochem. 58:913-921.
Wiggins, R. C. and Morell, P. 1980. Phosphorylation and fucosylation of myelin proteins in vitro by sciatic nerve from developing rats. J. Neurochem. 34:627-634.
Matthieu, J. M., Everly, J. R., Brady, R. O., and Quarles, R. H. 1975. [35]Sulfate incorporation into myelin glycoproteins. II. Peripheral nervous tissue. Biochim. Biophys. Acta 392:167-174.
Agrawal, H. C., Schmidt, R. E., and Agrawal, D. 1983. In vivo incorporation of [3H]palmitate into P0 protein, the major intrinsic protein of rat sciatic nerve myelin. J. Biol. Chem. 258: 6556-6560.
Trapp, B. D., Inoyama, Y., Sternberger, N. H., Quarles, R. H., and Webster, H. de F. 1981. Immunochemical localization of P0 in Golgi complex membranes and myelin of developing rat Schwann cells. J. Cell Biol. 90:1-6.
Kirschner, D. A. and Ganser, A. L. 1980 Compact myelin exists in the absence of myelin basic protein in the shiverer mutant mouse. Nature 283:207-210.
Lees, M. B. and Brostoff, S. W 1984. Proteins of myelin, pages 197-204, in Morell, P., (ed.), Myelin, Plenum Press, New York.
Uyemura, K., Kitamura, K, and Miura, M. 1992. Structure and molecular biology of P0 protein, pages 481-508, in Martenson, R. E. (ed.), Myelin: Biology and Chemistry, CRC Press, Boca Raton, FL.
Spiryda, L. B. 1998. Myelin protein zero and membrane adhesion. J. Neurosci. Res. 54:137-146.
Sakamoto, Y., Kitamura, K., Yoshimura, K., Nishijima, T., and Uyemura, K. 1987. Complete amino acid sequence of P0 protein in bovine peripheral nerve myelin. J. Biol. Chem. 262:4208-4214.
Lemke, G. and Axel, R. 1985. Isolation and sequence of a cDNA encoding the major structural protein of peripheral myelin. Cell 40:501-508.
Lemke, G., Lamar, E., and Patterson, J. 1988. Isolation and analysis of the gene encoding peripheral myelin protein zero. Neuron 1:75-83.
Bollensen, E. and Schachner, M. 1987. The peripheral myelin glycoprotein P0 expresses the L2/HNK-1 and L3 carbohydrate structures shared by neural adhesion molecules. Neurosci. Lett. 82:77-82.
Voshol, H., van Zuylen, C. W. E. M., Orberger, G., Vliegenthart, J. F. G., and Schachner, M. 1996. Structure of the HNK-1 carbohydrate epitope on bovine peripheral myelin glycoprotein P0. J. Biol. Chem. 271:22957-22960.
D'Urso, D., Brophy, P. J., Staugaitis, S. M., Gillespie, S., Frey, A. B., Stempak, J. G., and Colman, D. R. 1990. Protein zero of peripheral nerve myelin: biosynthesis, membrane insertion, and evidence for homotypic interaction. Neuron 2:449-460.
Filbin, M. T., Walsh, F. S., Trapp, B. D., Pizzey, J. A., and Tennekoon, G. I. 1990. Role of myelin P0 protein as a homophilic adhesion molecule. Nature 344:871-872.
Schneider-Schaulies, J. U., von Brunn, A., and Schachner, M. 1990. Recombinant peripheral myelin protein P0 confers both adhesion and neurite outgrowth-promoting properties. J. Neurosci. Res. 27:286-297.
Shapiro, L., Doyle, J. P., Hensley, P., Colman, D. R., and Hendrickson, W. 1996. Crystal structure of the extracellular domain from P0, the major structural protein of peripheral nerve myelin. Neuron 17:435-449.
Inouye, H., Tsuruta, H., Sedzik, J., Uyemura, K., and Kirschner, D. A. 1999. Tetrameric assembly of full-sequence protein zero myelin glycoprotein by synchrotron x-ray scattering. Biophys. J. 76:423-437.
Thompson, A. J., Cronin, M. S., and Kirschner, D. A. 2002. Myelin protein zero exists as dimers and tetramers in native membranes of Xenopus laevis peripheral nerve. J. Neurosci. Res. 67:766-771.
D Urso, D., Ehrhardt, P., and Muller, H. W. 1999. Peripheral myelin protein 22 and protein zero: a novel association in peripheral nervous system myelin. J. Neurosci. 19:3396-3403.
Griffith, L. S., Schmitz, B., and Schachner, M. 1992. L2/HNK-1 carbohydrate and protein-protein interactions mediate the homophilic binding of the neural adhesion molecule P0. J. Neurosci. Res. 33:639-648.
Filbin, M. T. and Tennekoon, G. 1993. Homophilic adhesion of the myelin P0 protein requires glycosylation of both molecules in the homophilic pair. J. Cell Biol. 122:451-459.
Filbin, M. T., Zhang, K., Li, W., and Gao, Y. 1999. Characterization of the effect on adhesion of different mutations in myelin P0 protein. Ann. N. Y. Acad. Sci. 883:160-167.
Zhang, K. and Filbin, M. T. 1994. Formation of a disulfide bond in the immunoglobulin domain of the myelin P0 protein is essential for its adhesion. J. Neurochem. 63:367-370.
Zhang, K. and Filbin, M. T. 1998. Myelin P0 protein mutated at cys21 has a dominant-negative effect on adhesion of wild type P0. J. Neurosci. Res. 53:1-6.
Ding, Y. and Brunden, K. R. 1994. The cytoplasmic domain of myelin glycoprotein P0 interacts with negatively charged phospholipid bilayers. J. Biol. Chem. 269:10764-10770.
Wong, M.-H. and Filbin, M. T. 1994. The cytoplasmic domain of the myelin P0 protein influences the adhesive interactions of its extracellular domain. J. Cell Biol. 126:1089-1097.
Wong, M.-H. and Filbin, M. T. 1996. Dominant-negative effect on adhesion by myelin P0 protein truncated in its cytoplasmic domain. J. Cell Biol. 134:1531-1541.
Lanwert, C. and Jeserich, G. 2001. Structure, heterologous expression, and adhesive properties of the P0-like myelin glycoprotein IP1 of trout CNS. Microsc. Res. Technol. 52:637-644.
Xu, W., Shy, M., Kamholz, J., Elferink, L., Xu, G., Lilien, J., and Balsamo, J. 2001. Mutations in the cytoplasmic domain of P0 reveal a role for PKC-mediated phosphorylation in adhesion and myelination. J. Cell Biol. 155:439-446.
Bizzozero, O. A., Fridal, K., and Pastuszyn, A. 1994. Identification of the palmitoylation site in rat myelin P0 glycoprotein. J. Neurochem. 62:1163-1171.
Gao, Y., Li, W., and Filbin, M. T. 2000. Acylation of myelin P0 protein is required for adhesion. J. Neurosci. Res. 60:704-713.
Eichberg, J. and Iyer, S. 1996. Phosphorylation of myelin proteins: recent advances. Neurochem. Res. 21:257-535.
Brunden, K. R. and Poduslo, J. F. 1987. A phorbol estersensitive kinase catalyzes the phosphorylation of P0 glycoprotein in myelin. J. Neurochem. 49:1863-1872.
Agrawal, H. C. and Agrawal, D. 1989. Tumor promoters accentuate phosphorylation of P0: evidence for the presence of protein kinase C in purified PNS myelin. Neurochem. Res. 14:409-413.
Rowe-Rendleman, C. R. and Eichberg, J. 1994. P0 phosphorylation in nerves from normal and diabetic rats: role of protein kinase C and turnover of phosphate groups. Neurochem. Res. 19:1023-1031.
Suzuki, M., Sakamoto, Y., Kitamura, K., Fukunuga, K., Yamamoto, H., Miyamoto, E., and Uyemura, K. 1990. Phosphorylation of P0 glycoprotein in peripheral nerve myelin. J. Neurochem. 55:1966-1971.
Borghini, I., Ania-Laherta, A., Regazzi, R., Ferrari, G., Gjinovci, A., Wollheim, C. B., and Pralong, W. F. 1994. Alpha, beta I, beta II, delta and epsilon protein kinase C isoforms and compound activity in the sciatic nerve of normal and diabetic rats. J. Neurochem. 62:686-696.
Iyer, S., Rowe-Rendleman, C. L., Bianchi, R., and Eichberg, J. 1996. Tyrosine phosphorylation of myelin protein P0. J. Neurosci. Res. 46:531-539.
Iyer, S., Bianchi, R., and Eichberg, J. 2000. Tyrosine phosphorylation of PNS myelin P0 occurs in the cytoplasmic domain and is maximal during early development. J. Neurochem. 75:347-354.
Xu, W., Zhao, R., Sui, X., Xu, F., and Zhao, Z. J. 2000. Tyrosine phosphorylation of myelin P0 and its implication in signal transduction. Biochem. Biophys. Res. Commun. 267:820-825.
Warner, L. E., Hilz, M. J., Appel, S. H., Killian, J. M., Kolodny, E. H., Karpati, G., Carpenter, S., Walters, G. V., Wheeler, C., Witt, D., Bodell, A., Selis, E., Van Broeckhoven, C., and Lupski, J. R. 1996. Clinical phenotypes of different MPZ (P0) mutations may include Charcot-Marie-Tooth type 1B, Dejerine-Sottas and congenital hypomyelination. Neuron 17:451-460.
Warner, L. E., Garcia, C. A., and Lupski, J. R. 1999. Hereditary peripheral neuropathies: clinical forms, genetics and molecular mechanisms. Annu. Rev. Med. 50:263-275.
Previtali, S. C., Quattrini, A., Fasolini, M., Panzeri, M. C., Villa, A., Filbin, M. T., Li, W., Chiu, S.-Y., Messing, A., Wrabetz, L., and Feltri, M. L. 2000. Epitope-tagged P0 glycoprotein causes Charcot-Marie-Tooth-like neuropathy in transgenic mice. J. Cell Biol. 151:1035-1045.
Senderek, J., Hermanns, B., Lehmann, U., Bergmann, C., Marx, G., Kabus, C., Timmerman, V., Stottenberg-Didinger, G., and Schroder, J. M. 2000. Charot-Marie-Tooth neuropathy type 2 and P0 point mutations: two novel amino acids substitutions (asp61gly; try119cys) and a possible “hotspot” on thr124met. Brain Pathol. 10:235-248.
Berger, P., Young, P., and Suter, U. 2002. Molecular cell biology of Charcot-Marie-Tooth disease. Neurogenetics 4:1-15.
Bolino, A., Muglia, M., Conforti, F. L., LeGuern, E., Salih, M. A., Georgiou, D. M., Christodoulou, K., Hausmanowa-Petrusewixz, I., Mandich, P., Schenone, A., Gambardella, A., Bono, F., Quattrone, A., DeVoto, M., and Monaco, A. P. 2000. Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat. Genet. 25:17-19.
Houldin, H., King, R. H., Wood, N. W., Thomas, P. K., and Reilly, M. M. 2001. Mutations in the 5' region of the myotubularin-related protein 2 (MTMR2) gene in autosomal recessive hereditary neuropathy with focally folded myelin. Brain 124:907-915.
Berger, P., Bonneick, S., Willi, S., Wymann, M., and Suter, U. 2002. Loss of phosphatase activity in myotubularin-related protein 2 is associated with Charcot-Marie-Tooth disease type 4B1. Hum. Mutat. 15:1569-1579.
Bolino, A., Marigo, V., Ferrera, F., Loader, J., Romio, L., Leoni, A., Di Duca, M., Cinti, R., Cecchi, C., Feltri, M. L., Wrabetz, L., Ravazzolo, R., and Monaco, A. P. 2002. Molecular characterization and expression analysis of Mtmr2, a mouse homologue of MTMR2, the Myotubularin-related 2 gene, mutated in CMT4B. Gene 283:17-26.
Nakagawa, M., Suchara, M., Saito, A., Takashima, H., Umchara, F., Saito, M., Kanzato, N., Matsuzaki, T., Takenaga, S., Sakoda, S., Izumo, S., and Osame, M. 1999. A novel MPZ gene mutation in dominantly inherited neuropathy with focally folded myelin sheaths. Neurology 52:1271-1275.
Fabrizi, G. M., Taioli, F., Cavallaro, T., Rigatelli, F., Simonati, A., Mariani, G., Perrone, P., and Rizzuto, N. 2000. Focally folded melin in Charcot-Marie-Tooth neuropathy type 1B with ser49leu in the myelin protein zero. Acta Neuropathol. (Berl.) 100:299-304.
Brunden, K. R. 1992. Age-dependent changes in the oligosaccharide structure of the major myelin glycoprotein, PO. J. Neurochem. 58:1659-1666.
Trapp, B. D., Kidd, G. J., Hauer, P., Mulrenin, E., Haney, C. A., and Andrews, S. B. 1995. Polarization of myelinating Schwann cell surface membranes: Role of microtubules and the trans-Golgi network. J. Neurosci. 15:1797-1807.
Pfend, G., Matthieu, J.-M., Garin, N., and Tosic, M. 2001. Implication of the extracellular disulfide bond on myelin protein zero expression. Neurochem. Res. 26:503-510.
Boll, W., Ohno, H., Songyang, Z., Rapport, I., and Cantley, L. C. 1996. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 15:5789-6795.
Owens, D. J. and Evans, P. R. 1998. A structural explanation for the recognition of tyrosine-based endocytic signals. Science 282:1327-1332.
Yin, X., Kidd, G. J., Wrabetz, L., Feltri, M. L., Messing, A., and Trapp, B. D. 2000. Schwann cell myelination requires timely and precise targeting of P(0) protein. J. Cell Biol. 148: 1009-1020.
Wrabetz, L., Feltri, M. L., Quattrini, A., Imperiale, D., Previtali, S., Antonio, M., Martini, R., Yin, X., Trapp, B. D., Zhou, L., Chiu, S. Y., and Messing, A. 2000. P(0) glycoprotein over-expression causes congenital hypomyelination of peripheral nerves. J. Cell Biol. 148:1021-1034.
Notterpek, L., Snipes, G. J., and Shooter, E. M. 1999. Temporal expression pattern of peripheral myelin protein in 22 during in vivo and in vitro myelination. Glia 25:358-369.
Kamholz, J., Awatramanai, R., Menichella, D., Jiang, H., Xu, W., and Shy, M. 1999. Regulation of myelin-specific gene expression: relevance to CMT1. Ann. N.Y. Acad. Sci. 883:91-108.
Giese, K. P., Martin, R., Lemke, G., Soriano, P., and Schachner, M. 1992. Mouse P0 gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell 71:565-576.
Martini, R., Mohajeri, M. H., Kasper, S., Giese, K. P., and Schachner M. 1995. Mice doubly deficient in the genes for P0 and myelin basic protein show that both proteins contribute to the formation of the major dense line in peripheral nerve myelin. J. Neurosci. 15:4488-4495.
Martini, R. and Schachner, M. 1997. Molecular bases of myelin formation as revealed by investigations on mice deficient in glial cell surface molecules. Glia 19:298-310.
Martini, R., Zielasek, J., Toyka, K. V., Giese, K. P., and Schachner, M. 1995. Protein zero (P0)-deficient mice show myelin degeneration in peripheral nerves characteristic of inherited human neuropathies. Nat. Genet. 11:281-286.
Xu, W., Manichella, D., Jiang, H., Vallat, J.-M., Lilien, J., Baron, P., Scarlato, G., Kamholz, J., and Shy, M. E. 2000. Absence of P0 leads to the dysregulation of myelin gene expression and myelin morphogenesis. J. Neurosci. Res. 60:714-724.
Fannon, A. M., Sherman, D. L., Ilyina-Gragerova, G., Brophy, P. J., Friedrich, V. L., Jr., and Colman, D. R. 1995. Novel E-cadherin-mediated adhesion in peripheral nerve: Schwann cell architecture is stabilized by autotypic adherens junctions. J. Cell Biol. 129:189-202.
Menichella, D. M., Arroyo, E. J., Awatramani, R., Xu, T., Baron, P., Vallat, J. M., Balsamo, J., Lilien J., Scarlato, G., Kamholz, J., Schere, S. S., and Shy, M. 2001. Protein zero is necessary for E-cadherin-mediated adherens junction formation in Schwann cells. Mol. Cell. Neurosci. 18:606-618.
Lee, M. J., Brennan, A., Blanchard, A., Zoidl, G., and Dong, Z. 1997. P0 is constitutively expressed in the rat neural crest and embryonic nerves and is negatively and positively regulated by axons to generate non-myelin-forming and myelin-forming Schwann cells, respectively. Mol. Cell. Neurosci. 8:336-350.
Brown, A. M. and Lemke, G. 1997. Multiple regulatory elements control transcription of the peripheral myein protein zero gene. J. Biol. Chem. 272:28939-28947.
Kuhlbrodt, K., Herbarth, B., Sock, E., Enderich, J., Hermans-Borgmeyer, J., and Wegner, M. 1998. Cooperative function of POU proteins and SOX proteins in glial cells. J. Biol. Chem. 273:16050-16057.
Peirano, R. I., Goerich, D. E., Riethmacher, D., and Wegner, M. 2000. Protein zero gene expression is regulated by the glial transcription factor Sox 10. Mol. Cell. Biol. 20:3198-3209.
Peirano, R. I. and Wegner, M. 2000. The glial transcription factor Sox10 binds to DNA both as monomer and dimmer with different functional consequences. Nucleic Acids Res. 28:3047-3055.
Morgan, L., Kristjan, R., Jessen, K. R., and Mirsky, R. 1994. Negative regulation of the P0 gene in Schwann cells: suppression of P0 mRNA and protein induction in cultured Schwann cells by FGF2 and TGFb1, TGFb2 and TGFb3. Development 120:1399-1409.
Stewart, H. J., Bradke, F., Tabernero, A., Morrell, D., Jessen, K. R., and Mirsky, R. 1996. Regulation of rat Schwann cell Po expression and DNA synthesis by insulin-like growth factors in vivo. Eur. J. Neurosci. 8:553-564.
Russell, J. W., Cheng, H. L., and Golovou, D. 2000. Insulin-like growth factor-I promotes myelination of peripheral sensory axons. J. Neuropathol. Exp. Neurol. 59:575-584.
Einheber, S., Hannocks, M. J., Metz, C. N., Rifkin, D. B., and Salzer, J. L. 1995. Transforming growth factor-beta 1 regulates axon/Schwann cell interactions. J. Cell Biol. 129:443-458.
Guenard, V., Gwynn, L. A., and Wood, P. M. 1995. Transforming growth factor-beta blocks myelination but not ensheathment of axons by Schwann cells in vitro. J. Neurosci. 15:419-428.
Koenig, H., Schumacher, M., Ferzaz, B., Do Thi, A. N., Ressouches, A., Guennoun, R., Jung-Testas, I., Robel, P. Akwa, Y., and Baulieu, E. E. 1995. Progesterone synthesis and myelin formation by Schwann cells. Science 268:1500-1503.
Schumacher, M., Guennoun, R., Mercier, G., Desarnaud, F., Lacor, P., Benavides, J., Ferzaz, B., Robert, F., and Baulieu, E. E. 2001. Progesterone synthesis and myelin formation in peripheral nerves. Brain Res. Rev. 37:343-359.
Magnaghi, V., Cavaretta, I., Galbiati, M., Martini, L., and Melcangi, R. C. 2001. Neuroactive steroids and peripheral myelin proteins. Brain Res. Rev. 37:360-371.
Desarnaud, F., Do Thi, A. N., Brown, A. M., Lemke, G., Suter, U., Baulieu, E. E., and Schumacher, M. 1998. Progesterone stimulates the activity of the promoters of peripheral myelin protein-22 and protein zero genes in Schwann cells. J. Neurochem. 71:1765-1768.
Melcangi, R. C., Magnaghi, V., Cavaretta, I., Zucchi, I., Bovolin, P., D Urso, D., and Martini, L. 1999. Progesterone derivatives are able to influence peripheral myelin protein 22 and P0 gene expression: possible mechanisms of action. J. Neurosci. Res. 56:349-357.
Melcangi, R. C., Magnaghi, V., Cavarretta, I., Martini, L., and Piva, F. 1998. Age-induced decrease of glycoprotein P0 and myelin basic protein gene expression in the rat sciatic nerve. repair by steroid derivative. Neuroscience 85:569-578.
Robert, F., Guennown, R., Desarnaud, F., Do-Thi, A., Benmessahel, Y., Baulieu, E. E., and Schumacher, M. 2001. Synthesis of progesterone in Schwann cells: regulation by sensory neurons. Eur. J. Neurosci. 13:916-924.
Magnaghi, V., Cavarretta, I., Zucchi, I., Susani, L., Rupprecht, R., Hermann, B., Martini, L., and Melcangi, R. C. 1999. P0 gene expression is modulated by androgens in the sciatic nerve of adult male rats. Mol. Brain Res. 70:36-44.
Desarnaud, F., Bidichandani, S., Patel, P. I., Baulieu, E. E., and Schumacher, M. 2000. Glucocorticosteroids stimulate the activity of the promoters of peripheral myelin protein-22 genes in Schwann cells. Brain Res. 865:12-16.
Donaghy, M., Sisodiya, S. M., Kennett, R., McDonald, B., Haites, N., and Bell, C. 2000. Steroid responsive polyneuropathy in a family with a novel myelin protein zero mutation. J. Neurol. Neurosurg. Psychiatry 69:799-805.
Zhang, S.-M., Marsh, R., Rainer, N., and Brackenbury, R. 1995. Myelin glycoprotein P0 is expressed at early stages of chicken and rat embryogensis. J. Neurosci. Res. 40:241-250.
Lee, M. J., Calle, E., Brennan, A., Ahmed, S., Sviderskaya, E., Jessen, K. R., and Mirsky, R. 2001. In early development of the rat mRNA for the major myelin protein P(0) is expressed in nonsensory areas of the embryonic inner ear, notochord, enteric nervous system, and olfactory ensheathing cells. Dev. Dyn. 222:40-51.
Hagedorn, L., Suter, U., and Sommer, L. 1999. P0 and PMP22 mark a multipotent neural crest cell type that displays community effects in response to TGF-β family factors. Development 126:3781-3794.
Gallego, R. G., Jimenez-Blanco, J. L., Thijssen-van Zuylen, C. W. E. M., Gotfredsen, C. H., Voshol, H., Duus, J. O., Schachner, M., and Vliegenthart, J. F. G. 2001. Epitope diversity of N-glycans from bovine peripheral myelin glycoprotein P0 revealed by mass spectrometry and nano probe magic angle spinning 1H NMR spectroscopy. J. Biol. Chem. 276:30834-30844.
Sommer, L. and Suter, U. 1998. The glycoprotein P0 in peripheral gliogenesis. Cell Tissue Res. 292:11-16.
Yazaki, T., Miura, M., Asou, H., Toya, S., and Uyemura, K., 1991. Myelin P0 protein expressed in C6 cells promote neurite outgrowth. Biomed. Res. 12:223-230.
Yazaki, T., Miura, M., Asou, H., Toya, S., and Uyemura, K. 1994. Peripheral myelin P0 protein mediates neurite outgrowth of cortical neurons in vitro and axonal regeneration in vivo. Nuerosci. Lett. 17:13-16.
Samsam, M., Frei, R., Marziniak, M., Martini, R., and Sommer, C. 2002. Impaired sensory function in heterozygous P0 knockout mice is associated with nodal changes in sensory nerves. J. Neurosci. Res. 67:167-173.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eichberg, J. Myelin P0: New Knowledge and New Roles. Neurochem Res 27, 1331–1340 (2002). https://doi.org/10.1023/A:1021619631869
Issue Date:
DOI: https://doi.org/10.1023/A:1021619631869