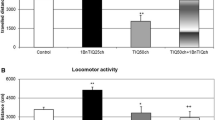
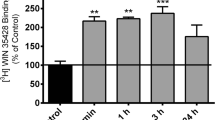
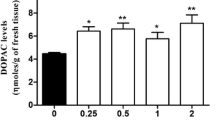
Abstract
The DOPAC/DA ratio in mouse striatum, in striatal synaptosomes, and in rat urine after MPP+ and MPTP neurotoxin administrations to the animals was followed temporally. The neurotoxins were given intraperitoneally and, in some experiments, to enhance the sensitivity, the animals were subsequently reserpinized before either sacrifice or 24 hour urine collection. MPP+ treatment, followed by saline, weakly lowered mouse striatal DOPAC/DA ratio up to 6 hours; in reserpinized animals, however, the neurotoxin reduced striatal ratio potently and for longer periods. Similarly, MPP+ reduced rat (saline treated) urinary DOPAC level and DOPAC/DA ratio in the short term (1.0 hr) while the neurotoxin effects could still be detected following longer periods up to 27 days in reserpinized animals. A single MPTP treatment (90 min.), followed by preparation of striatal synaptosomal fraction and its incubation (37°C) with or without reserpine, also led to a reduced DOPAC/DA ratio. Although mainly the pooled peripheral effect is directly indicated by urinary DOPAC/DA ratio, MPP+ may reduce DA oxidation in the CNS and may similarly affect the amine oxidation in the peripheral tissues. The CNS and peripheral effects differ, however, in respect to dose-sensitivity and time course. The similarities between the CNS and peripheral effects suggest that a blunted rise of urinary DOPAC/DA ratio after reserpine challenge could be utilized as a peripheral marker of MPP+ action in the CNS, a marker that is not currently available.
Similar content being viewed by others
REFERENCES
Davis, G. C., Williams, A. C., Markey, S. P., Ebert, M. H., Caine, E. D., Reichert, C. M., and Kopin, I. J. 1979. Chronic Parkinsonism secondary to intravenous injection of meperidine analogs. Psychiat Res. 1:249-254.
Burns, R. S., Chiueh, C. C., Markey, S. P., Ebert, M. H., Jacobowitz, D. M., and Kopin, I. P. 1983. A primate model of parkinsonism: Selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc. Natl. Acad. Sci. 80:4546-4550.
Jenner, P., Rupniak, N. M. J., Rose, J., Kelly, E., Kilpatrick, G., Lees, A., and Marsden, C. D. 1984. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in the common marmoset. Neurosci. Lett. 50:85-90.
Langston, J. W., Forno, L. S., Rebert, C. S., and Irwin, I. 1984. Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the squirrel monkey. Brain Res. 292:390-394.
Heikkila, R. E., Manzino, L., Cabbat, F. S., and Duvoisin, R. C. 1984. Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by monoamine oxidase inhibitors. Nature 311:467-469.
Markey, S. P., Johannessen, J. N., Chiueh, C. C., Burns, R. S., and Harkenham, M. A. 1984. Intraneuronal generation of a pyridinium metabolite may cause drug-induced Parkinsonism, Nature 311:464-467.
Rollema, H., Damsma, G., Horn, A. S., Devries, J. B., and Westerink, B. H. C. 1986. Brain dialysis in conscious rats reveals an instantaneous massive release of striatal dopamine in response to MPP+. Eur. J. Pharmacol. 126:345-346.
Caliguri, E. J., and Johannessen, J. N. 1991. Selective decrease in extracellular DOPAC concentrations in rat striataum following in vivo dialysis with low concentrations of MPP+. Brain Res. 548:94-99.
Bagchi, S. P. 1991. Reserpine induced intraneuronal dopamine oxidation: reversal by MPP+ action. Life Sci. 48:1007-1013.
Bagchi, S. P. 1992. Trace dosages of the neurotoxins MPTP and MPP+ may affect brain dopamine in vivo. Life Sci. 51:389-396.
Tipton, K. F., and Singer, T. P. 1993. Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J. Neurochem 61:1191-1206.
Favre, R., deHaut, M., Dalmaz, Y., Pequignot, J. M., and Peyrin, L. 1986. Peripheral distribution of free dopamine and its metabolites in the rat. J. Neural. Trans. 66:135-149.
Glavin, G. B. 1992. Dopamine: a stress modulator in the brain and gut. Gen. Pharmacol. 23:1023-1026.
Amenta, F., Gallo, P., Rossodivita, A., and Ricci, A. 1993. Radioligand bindings and autoradiographic analysis of dopamine receptors in the human heart. Naunyn-Schmiedeberg's Arch. Pharmacol. 347:147-154.
Kujacic, M., and Carlsson, A. 1993. Evidence for an increased catecholamine synthesis in rat adrenal glands following stimulation of peripheral dopamine receptors. J. Neural. Transm. 92:73-79.
Hoeldtke, R., Rogawski, M., and Wurtman, R. J. 1974. Effect of selective destruction of central and peripheral catecholamine-containing neurones with 6-hydroxydopamine on catecholamine excretion in the rat. Br. J. Pharmacol. 50:265-270.
Peyrin, L., Simon, H., Cottet-Emard, J. M., Bruneau, N., and Le Moal, M. 1982. 6-hydroxydopamine lesions of dopaminergic A10 neurons. Long-term effects on the urinary excretion of free and conjugated catecholamines and their metabolites in the rat. Brain Res. 235:363-369.
Edwards, D. J., Ravitch, J., Knopf, S., and Sedlock, M. L. 1985. Effects of intraventricular injections of 6-hydroxydopamine on amine metabolites in rat brain and urine. Biochem. Pharmacol. 34:1255-1263.
Bagchi, S. P. 1987. Single-step method for derivatization of dopamine and some related compounds in aqueous media for gas chromatography. J. Chromatograph. 421:227-235.
Kujacic, M., and Carlsson, A. 1994. Effect of MPP+ on catecholamine levels in adrenal glands and heart of rats. Naunyn-Schmiedebergs Arch. Pharmacol. 350:245-251.
Snape, B. M., Pileblad, E., Ekman, A., Magnusson, T., Carlsson, T., and Engel, J. 1988. The effect of 1-methyl-4-phenylpyridium ion (MPP+) on the efflux and metabolism of endogenous dopamine in rat striatal slices. J. Pharm. Pharmacol. 40:620-626.
Zetterstrom, T., Sharp, T., Collin, A. K., and Understedt, U. 1988. In vivo measurement of extracellular dopamine and DOPAC in rat striatum after various dopamine-releasing drugs; implications for the origin of extracellular DOPAC. Eur. J. Pharmacol. 148:327-334.
Del Zompo, M., Piccardi, M. P., Ruiu, S., Quartu, M., Gessa, G. L., and Vaccari, A. 1993. Selective MPP+ uptake into synaptic dopamine vesicles: possible involvement in MPTP neurotoxicity. Br. J. Pharmacol. 109:411-414.
Youngster, S. K., McKeown, K. A., Jin, Y. Z., Ramsay, R. R., Heikkila, R. E., and Singer, T. P. 1989. Oxidation of analogs of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by monoamine oxidase A and B and the inhibition of monoamine oxidases by the oxidation products. J. Neurochem. 53:1837-1842.
Youngster, S. K., Nicklas, W. J., and Heikkila, R. E. 1989. Structure-activity study of the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity. II. Evaluation of the biological activity of the pyridinium metabolites formed from the monoamine oxidase-catalyzed oxidation of MPTP analogs. J. Pharmacol. Expt. Ther. 249:829-835.
Rollema, H., Kuhr, W. G., Kranenborg, G., de Vries, J., and Van Den Berg, C. 1988. MPP+-induced efflux of dopamine and lactate from rat striatum have similar time courses as shown by in vivo brain dialysis. J. Pharmacol. Exp. Ther. 245:858-866.
Chiueh, C. C., Miyake, H., Huang, S. J., and Peng, M. T. 1990. Role of extracellular calcium ion on 1-methyl-4-phenylpyridine induced sustained exocytosis of endogenous striatal dopamine in vivo. Eur. J. Pharmacol. 183:1069-1070.
Trendelenberg, U. 1979. The release of catecholamines from adrenergic neurones. Paton, D. M. (ed) 333-354, Pergamon Press, New York.
Desole, M. S., Esposito, G., Fresu, L., Migheli, R., Enrico, P., Miele, M., DeNatale, G., and Miele, G. 1993. Correlation between 1-methyl-4-phenylpyridinium ion (MPP+) levels, ascorbic acid oxidation and glutathione levels in the striatal synaptosomes of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated rat. Neurosci. Lett. 161:121-123.
Fuller, R. W., Hemrick-Luecke, S. K., and Perry, K. W. 1989. Tissue concentrations of MPTP and MPP+ in relation to catecholamine depletion after the oral or subcutaneous administration of MPTP to mice. Life Sci. 45:2077-2083.
Irwin, I., DeLanney, L. E., DiMonte, D., and Langston J. W. 1989. The biodisposition of MPP+ in mouse brain. Neurosci. Lett. 101:83-88.
Melamed, E., Rosenthal, J., Cohen, O., Uzzan, A., and Globus, M. 1985. Amphetamine, but not reserpine, protects mice against dopaminergic neurotoxicity of MPTP. Neuropharmacology 24:923-925.
Reinhard Jr., J. F., Daniels, A. J., and Viveros, O. H. 1988. Potentiation by reserpine and tetrabenazine of brain catecholamine depletions by MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) in the mouse, evidence for subcellular sequestration as basis for cellular resistance to the toxicant. Neurosci. Lett. 90:349-353.
Reinhard Jr., J. F., Carmichael, S. W., and Daniels, A. J. 1990. Mechanisms of toxicity and cellular resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium in adrenomedullary chromaffin cell culture. J. Neurochem. 55:311-320.
Johannessen, J. N., Chiueh, C. C., Bacon, J. B., Garrick, N. A., Burns, R. S., Weise, V. K., Kopin, I. J., Parisi, J. E., and Markey, S. P. 1989. Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the dog: effect of pargyline treatment. J. Neurochem. 53:582-589.
Roth, R. H., Murrin, L. C., and Walters, J. R. 1976. Central dopaminergic neurones: effect of alterations in impulse flow. Eur. J. Pharmacol. 36:163-171.
Del Zompo, M., Piccardi, M. P., Ruiu, S., Corsini, G. U., and Vaccari, A. 1991. High-affinity binding of [3H]1-methyl-4-phenyl-2,3-dihydropyridinium ion to mouse striatal membranes: putative vesicular location. Eur. J. Pharmacol. 202:293-294.
Chiueh, C. C., Miyake, H., and Peng, M. T. 1993. Role of dopamine autooxidation, hydroxyl radical generation, and calcium overload in underlying mechanisms involved in MPTP-induced Parkinsonism. Adv. Neurol. 60:251-258.
Ziv, I., Melamed, E., Nardi, N., Luria, D., Achiron, A., Offen, D., Barzilai, A. 1994. Dopamine induces apoptosis-like cell death in cultured chick sympathetic neurons—a possible novel pathogenetic mechanism in Parkinson's disease. Neurosci. Lett. 170:136-140.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bagchi, S.P. Striatal and Urinary DOPAC/DA Ratio May Indicate a Long-Lasting DA Release Enhancement by MPP+ and MPTP. Neurochem Res 23, 127–134 (1998). https://doi.org/10.1023/A:1022464421655
Issue Date:
DOI: https://doi.org/10.1023/A:1022464421655