Abstract
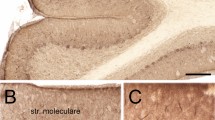
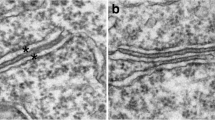
Cisternal stacks are induced during hypoxia, which may be associated with intracellular Ca2+ regulation. Although neurons are divided internally in different compartments, little is known about regional differences in cisternal stack formation. We investigated the effects of hypoxic hypoxia and later reoxygenation on cisternal stack formation and other ultrastructual changes in the proximal dendrite, dendritic spine, and cell body of cerebellar Purkinje cells in rats. After brief hypoxic events, cisternal stacks appeared predominantly in the proximal dendrites and after longer hypoxic events in dendritic spines and cell body. Following reoxygenation, cisternal stacks disappeared first in the cell body, followed by the dendritic spines, then the proximal dendrites. These results showed that stack formation occurred at different degrees and time courses among the three regions, and the effect was reversible, which suggests that these compartments are differentially sensitive to hypoxia.
Similar content being viewed by others
REFERENCES
Herndon, R. M. 1964. Lamellar bodies, an unusual arrangement of the granular endoplasmic reticulum. J. Cell Biol. 20:338-342.
Bestetti, G. and Rossi, G. L. 1980. The occurrence of cytoplasmic lamellar bodies in normal and pathologic conditions. Acta Neuropathol. (Berl.) 49:75-78.
Hansson, H. A. 1981. Lamellar bodies in Purkinje nerve cells experimentally induced by electric field. Brain Res. 216:187-191.
Palladini, G., Conforti, A., and Medolago-Albani, L. 1976. Ultrastructural hypoxic changes in Ammon's horn and Purkinje cells. Brain Res. 103:45-56.
Takei, K., Mignery, G. A., Mugnaini, E., Sudhof, T. C., and De Camilli, P. 1994. Inositol 1,4,5-trisphosphate receptor causes formation of ER cisternal stacks in transfected fibroblasts and in cerebellar Purkinje cells. Neuron 12:327-342.
Banno, T. and Kohno, K. 1996. Conformational changes of smooth endoplasmic reticulum induced by brief anoxia in rat Purkinje cells. J. Comp. Neurol. 369:462-471.
Rosenbluth, J. 1962. Subsurface cisterns and their relationship to the neuronal plasma membrane. J. Cell Biol. 13:405-421.
Nathaniel, E. J. H. and Nathaniel, D. R. 1966. Fine structure of the neurons of the posterior horn in the rat spinal cord. Anat. Res. 155:629-641.
Karlsson, U. and Schultz, R. L. 1966. Fixation of the central nervous system for electron microscopy by aldehyde perfusion. 3. Structual changes after exsanguination and delayed perfusion. J. Ultrastruct. Res. 14:47-63.
Van Nimwegen, D. and Sheldon, H. 1966. Early postmortem changes in cerebellar neurons of the rat. J. Ultrastruct. Res. 14:36-46.
Rossi, F., Cantino, D., and Strata, P. 1987. Morphology of Purkinje cell axon terminals in intracerebellar nuclei following inferior olive lesion. Neuroscience 22:99-112.
Choi, D. W. 1995. Calcium: Still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 18:58-60.
Miyakawa, H., Lev-Ram, V., Lasser-Ross, N., and Ross, W. N. 1992. Calcium transients evoked by climbing fiber and parallel fiber synaptic inputs in guinea pig cerebellar Purkinje neurons. J. Neurophysiol. 68:1178-1189.
Finch, E. A. and Augustine, G. J. 1998. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature 396:753-756.
Takechi, H., Eilers, J., and Konnerth, A. 1998. A new class of synaptic response involving calcium release in dendritic spines. Nature 396:757-760.
Berridge, M. J. 1998. Neuronal calcium signaling. Neuron 21:13-26.
Mignery, G. A., Sudhof, T. C., Takei, K., and De Camilli, P. 1989. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature 342:192-195.
Otsu, H., Yamamoto, A., Maeda, N., Mikoshiba, K., and Tashiro, Y. 1990. Immunogold localization of inositol 1, 4, 5-trisphosphate (InsP3) receptor in mouse cerebellar Purkinje cells using three monoclonal antibodies. Cell Struct. Funct. 15:163-173.
Satoh, T., Ross, C. A., Villa, A., Supattapone, S., Pozzan, T., Snyder, S. H., and Meldolesi, J. 1990. The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cells: Quantitative immunogold labeling reveals concentration in an ER subcompartment. J. Cell Biol. 111:615-624.
Takei, K., Stukenbrok, H., Metcalf, A., Mignery, G. A., Sudhof, T. C., Volpe, P., and De Camilli, P. 1992. Ca2+ stores in Purkinje neurons: Endoplasmic reticulum subcompartments demonstrated by the heterogeneous distribution of the InsP3 receptor, Ca(2+)-ATPase, and calsequestrin. J. Neurosci. 12:489-505.
Mitani, A., Yanase, H., Namba, S., Shudo, M., and Kataoka, K. 1995. In vitro ischemia-induced intracellular Ca2+ elevation in cerebellar slices: A comparative study with the values found in hippocampal slices. Acta Neuropathol. 89:2-7.
Banno, T. and Kohno, K. 1998. Conformational changes of the smooth endoplasmic reticulum are facilitated by L-glutamate and its receptors in rat Purkinje cells. J. Comp. Neurol. 402:252-263.
Ito, M. and Kano, M. 1982. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci. Lett. 33:253-258.
Konnerth, A., Llano, I., and Armstrong, C. M. 1990. Synaptic currents in cerebellar Purkinje cells. Proc. Natl. Acad. Sci. USA. 87:2662-2665.
Perkel, D. J., Hestrin, S., Sah, P., and Nicoll, R. A. 1990. Excitatory synaptic currents in Purkinje cells. Proc. Royal Soc. Lond. B. Biol. Sci. 241:116-121.
Martin, L. J., Blackstone, C. D., Huganir, R. L., and Price, D. L. 1992. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron 9:259-270.
Baude, A., Nusser, Z., Roberts, J. D., Mulvihill, E., McIlhinney, R. A., and Somogyi, P. 1993. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron 11:771-787.
Yuste, R. and Denk, W. 1995. Dendritic spines as basic functional units of neuronal integration. Nature 375:682-684.
Miyata, M., Finch, E. A., Khiroug, L., Hashimoto, K., Hayasaka, S., Oda, S. I., Inouye, M., Takagishi, Y., Augustine, G. J. and Kano, M. 2000. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron 28:233-244.
Harris, K. M. 1999. Structure, development, and plasticity of dendritic spines. Curr. Opin. Neurobiol. 9:343-348.
Hu, B. R., Park, M., Martone, M. E., Fischer, W. H., Ellisman, M. H., and Zivin, J. A. 1998. Assembly of proteins to postsynaptic densities after transient cerebral ischemia. J. Neurosci. 18:625-633.
Martone, M. E., Jones, Y. Z., Young, S. J., Ellisman, M. H., Zivin, J. A., and Hu, B. R. 1999. Modification of postsynaptic densities after transient cerebral ischemia: A quantitative and three-dimensional ultrastructural study. J. Neurosci. 19:1988-1997.
Martone, M. E., Hu, B. R., and Ellisman, M. H. 2000. Alterations of hippocampal postsynaptic densities following transient ischemia. Hippocampus 10:610-616.
Harris, K. M., Jensen, F. E., and Tsao, B. 1992. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: Implications for the maturation of synaptic physiology and long-term potentiation. J. Neurosci. 12:2685-2705.
Harris, K. M. and Kater, S. B. 1994. Dendritic spines: Cellular specializations imparting both stability and flexibility to synaptic function. Annu. Rev. Neurosci. 17:341-371.
Kennedy, M. B. 1994. The biochemistry of synaptic regulation in the central nervous system. Annu. Rev. Biochem. 63:571-600.
Suzuki, T. 1994. Protein kinases involved in the expression of long-term potentiation. Int. J. Biochem. 26:735-744.
Klauck, T. M. and Scott, J. D. 1995. The postsynaptic density: A subcellular anchor for signal transduction enzymes. Cell Signal. 7:747-757.
Chan, P. H. 1996. Role of oxidants in ischemic brain damage. Stroke 27:1124-1129.
Yoshimi, K., Takeda, M., Nishimura, T., Kudo, T., Nakamura, Y., Tada, K., and Iwata, N. 1991. An immunohistochemical study of MAP2 and clathrin in gerbil hippocampus after cerebral ischemia. Brain Res. 560:149-158.
Roberts-Lewis, J. M., Savage, M. J., Marcy, V. R., Pinsker, L. R., and Siman, R. 1994. Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J. Neurosci. 14:3934-3944.
Taylor, C. P. and Weber, M. L. 1993. Effect of temperature on synaptic function after reduced oxygen and glucose in hippocampal slices. Neuroscience 52:555-562.
Taylor, C. P., Weber, M. L., Gaughan, C. L., Lehning, E. J., and LoPachin, R. M. 1999. Oxygen/glucose deprivation in hippocampal slices: Altered intraneuronal elemental composition predicts structural and functional damage. J. Neurosci. 19:619-629.
Siesjo, B. K. 1992. Pathophysiology and treatment of focal cerebral ischemia. I. Pathophysiology. J. Neurosurg. 77:169-184.
Dykens, J. A. 1994. Isolated cerebral and cerebellar mitochondria produce free radicals when exposed to elevated Ca2+ and Na+: Implications for neurodegeneration. J. Neurochem. 63:584-591.
White, R. J. and Reynolds, I. J. 1997. Mitochondria accumulate Ca2+ following intense glutamate stimulation of cultured rat forebrain neurones. J. Physiol. 498:31-47.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikemoto, T., Yorifuji, H., Satoh, T. et al. Reversibility of Cisternal Stack Formation During Hypoxic Hypoxia and Subsequent Reoxygenation in Cerebellar Purkinje Cells. Neurochem Res 28, 1535–1542 (2003). https://doi.org/10.1023/A:1025674409572
Issue Date:
DOI: https://doi.org/10.1023/A:1025674409572