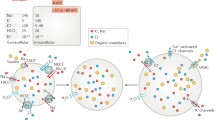
Abstract
Change in the intracellular concentration of osmolytes or the extracellular tonicity results in a rapid transmembrane water flow in mammalian cells until intracellular and extracellular tonicities are equilibrated. Most cells respond to the osmotic cell swelling by activation of volume-sensitive flux pathways for ions and organic osmolytes to restore their original cell volume. Taurine is an important organic osmolyte in mammalian cells, and taurine release via a volume-sensitive taurine efflux pathway is increased and the active taurine uptake via the taurine specific taurine transporter TauT decreased following osmotic cell swelling. The cellular signaling cascades, the second messengers profile, the activation of specific transporters, and the subsequent time course for the readjustment of the cellular content of osmolytes and volume vary from cell type to cell type. Using Ehrlich ascites tumor cells, NIH3T3 mouse fibroblasts and HeLa cells as biological systems, it is revealed that phospholipase A2-mediated mobilization of arachidonic acid from phospholipids and subsequent oxidation of the fatty acid via lipoxygenase systems to potent eicosanoids are essential elements in the signaling cascade that is activated by cell swelling and leads to release of osmolytes. The cellular signaling cascade and the activity of the volume-sensitive taurine efflux pathway are modulated by elements of the cytoskeleton, protein tyrosine kinases/phosphatases, GTP-binding proteins, Ca2+/calmodulin, and reactive oxygen species and nucleotides. Serine/threonine phosphorylation of the active taurine uptake system TauT or a putative regulator, as well as change in the membrane potential, are important elements in the regulation of TauT activity. A model describing the cellular sequence, which is activated by cell swelling and leads to activation of the volume-sensitive efflux pathway, is presented at the end of the review.
Similar content being viewed by others
REFERENCES
Hoffmann, E. K. and Dunham, P. B. 1995. Membrane mechanisms and intracellular signalling in cell volume regulation. Int. Rev. Cytol. 161:173–262.
Krogh, A. 1939. Osmotic regulation in aquatic animals. Dover Publication, Inc.
Bustamante, J., Lobo, M. V., Alonso, F. J., Mukala, N. T., Gine, E., Solis, J. M., Tamarit-Rodriguez, J., and Martin del Rio, R. 2001. An osmotic-sensitive taurine pool is localized in rat pancreatic islet cells containing glucagon and somatostatin. Am. J. Physiol. Endocrinol. Metab. 281:E1275–E1285.
Grunewald, R. W., Oppermann, M., Schettler, V., Fiedler, G. M., Jehle, P. M., and Schuettert, J. B. 2001. Polarized function of thick ascending limbs of Henle cells in osmoregulation. Kidney Int. 60:2290–2298.
Pasantes-Morales, H., Alavez, S., Sanchez Olea, R., and Moran, J. 1993. Contribution of organic and inorganic osmolytes to volume regulation in rat brain cells in culture. Neurochem. Res. 18: 445–452.
Roy, G. and Malo, C. 1992. Activation of amino acid diffusion by a volume increase in cultured kidney (MDCK) cells. J. Membr. Biol. 130:83–90.
Moran, J., Miranda, D., Pena-Segura, C., and Pasantes-Morales, H. 1997. Volume regulation in NIH/3T3 cells not expressing P-glycoprotein: II. Chloride and amino acid fluxes. Am. J. Physiol. 272:C1804–C1809.
Huxtable, R. J. 1992. Physiological actions of taurine. Physiol Rev. 72:101–163.
Hoffmann, E. K. and Lambert, I. H. 1983. Amino acid transport and cell volume regulation in Ehrlich ascites tumour cells. J. Physiol. 338:613–625.
Lang, F., Busch, G. L., Ritter, M., Volkl, H., Waldegger, S., Gulbins, E., and Haussinger, D. 1998. Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 78:247–306.
Pedersen, S. F., Mills, J. W., and Hoffmann, E. K. 1999. Role of the F-actin cytoskeleton in the RVD and RVI processes in Ehrlich ascites tumor cells. Exp. Cell Res. 252:63–74.
Marnela, K.-M., Timonen, M., and Lähdesmäki, P. 1984. Mass spectrometric analyses of brain synaptic peptides containing taurine. J. Neurochem. 43:1650–1653.
Kaya, K. and Sano, T. 1991. Definition of total biosynthesis pathway of taurolipids in Tetrahymena cells. Biochim. Biophys. Acta 1084:101–104.
Gaull, G. E. 1989. Taurine in pediatric nutrition: Review and update. Pediatrics 83:433–442.
Han, X., Budreau, A. M., and chesney, R. W. 2000. Identification of promoter elements involved in adaptive regulation of the taurine transporter gene: Role of cytosolic Ca2+ signaling. Adv. Exp. Med. Biol. 483:535–544.
Huxtable, R. J. and Sebring, L. A. 1986. Towards a unifying theory for the actions of taurine. Trends Pharmacol. Sci. 7:481–485.
Lombardini, J. B. 1991. Taurine: Retinal function. Brain Res. Brain Res. Rev. 16:151–169.
Kromphardt, H. 1965. On pH dependibility of transport of neutral amino acids in Ehrlich ascites tumor cells. Biochem. Z. 343: 283–293.
Lambert, I. H. 1984. Na-dependent taurine uptake in Ehrlich ascites tumor cells. Mol. Physiol. 6:233–246.
Lambert, I. H. 1985. Taurine transport in Ehrlich ascites tumour cells: Specificty and chloride dependence. Mol. Physiol. 7: 323–332.
Mollerup, J. and Lambert, I. H. 1998. Calyculin A modulates the kinetic constants for the Na+-coupled taurine transport in Ehrlich ascites tumour cells. Biochim. Biophys. Acta 1371:335–344.
Lill, H. and Nelson, N. 1998. Homologies and family relationships among Na+/Cl− neurotransmitter transporters. Methods Enzymol. 296:425–436.
Jhiang, S. M., Fithian, L., Smanik, P., McGill, J., Tong, Q., and Mazzaferri, E. L. 1993. Cloning of the human taurine transporter and characterization of taurine uptake in thyroid cells. FEBS Lett. 318:139–144.
Uchida, S., Kwon, H. M., Yamauchi, A., Preston, A. S., Marumo, F., and Handler, J. S. 1992. Molecular cloning of the cDNA for an MDCK cell Na+-and Cl−-dependent taurine transporter that is regulated by hypertonicity. Proc. Natl. Acad. Sci. USA 89: 8230–8234.
Vinnakota, S., Qian, X., Egal, H., Sarthy, V., and Sarkar, H. K. 1997. Molecular characterization and in situ localization of a mouse retinal taurine transporter. J. Neurochem. 69:2238–2250.
Takeuchi, K., Toyohara, H., and Sakaguchi, M. 2000. A hyperosmotic stress-induced mRNA of carp cell encodes Na+-and Cl−-dependent high affinity taurine transporter. Biochim. Biophys. Acta 1464:219–230.
Hruska, R. E., Padjen, A., Bressler, R., and Yamamura, H. I. 1978. Taurine: Sodium-dependent, high-affinity transport into rat brain synaptosomes. Mol. Pharmacol. 14:77–85.
Martin, D. L. and Shain, W. 1979. High affinity transport of taurine and beta-alanine and low affinity transport of gamma-aminobutyric acid by a single transport system in cultured glioma cells. J. Biol. Chem. 254:7076–7084.
Holopainen, I. and Kontro, P. 1984. Taurine and hypotaurine transport by a single system in cultured neuroblastoma cells. Acta Physiol Scand. 122:381–386.
Barakat, L., Wang, D., and Bordey, A. 2002. Carrier-mediated uptake and release of taurine from Bergmann glia in rat cerebellar slices. J. Physiol. 541:753–767.
Jones, D. P., Miller, L. A., and Chesney, R. W. 1993. Polarity of taurine transport in cultured renal epithelial cell lines: LLC-PK1 and MDCK. Am. J. Physiol. 265:F137–F145.
Wersinger, C., Rebel, G., and Lelong-Rebel, I. H. 2000. Detailed study of the different taurine uptake systems of colon LoVo MDR and non-MDR cell lines. Amino Acids 19:667–685.
Takahashi, K., Azuma, M., Yamada, T., Ohyabu, Y., Takahashi, K., Schaffer, S. W., and Azuma, J. 2003. Taurine transporter in primary cultured neonatal rat heart cells: A comparison between cardiac myocytes and nonmyocytes. Biochem. Pharmacol. 65: 1181–1187.
Keep, R. F. and Xiang, J. 1996. Choroid plexus taurine transport. Brain Res. 715:17–24.
Poulsen, K. A., Litman, T., Eriksen, J., Mollerup, J., and Lambert, I. H. 2002. Downregulation of taurine uptake in multidrug resistant Ehrlich ascites tumor cells. Amino Acids 22:333–350.
Wolff, N. A. and Kinne, R. 1988. Taurine transport by rabbit kidney brush-border membranes: Coupling to sodium, chloride, and the membrane potential. J. Membr. Biol. 102:131–139.
Miyamoto, Y., Tiruppathi, C., Ganapathy, V., and Leibach, F. H. 1989. Active transport of taurine in rabbit jejunal brush-border membrane vesicles. Am. J. Physiol. 257:G65–G72.
Turner, R. J. 1983. Quantitative studies of cotransport systems: Models and vesicles. J. Membr. Biol. 76:1–15.
Harvey, B. J. and Ellory, J. C. 1988. Stoechiometry of taurine transport by the β-system in pigeon erythrocytes. Biochem. Soc. Trans. 16:555–556.
Preston, R. L. and Chen, C. W. 1989. Inhibition of sodium-dependent taurine transport in red blood cells from the marine polychaete, Glycera dibranchiata, after exposure to mercury. Bull. Environ. Contam. Toxicol. 42:620–627.
Thoroed, S. M. and Fugelli, K. 1993. Characterization of the Na-dependent taurine influx in flounder erythrocytes. J. Comp. Physiol. 163:307–316.
Sakai, S., Tosake, T., Tasaka, J., Hashigushi, T., and Yoshihama, I. 1989. Taurine uptake by glial cells in the bullfrog symphathetic ganglia. Neurochem. Int. 14:193–198.
Stein, W. D. 1986. Intrinsic, apparent, and effective affinities of co-and countertransport systems. Am. J. Physiol. 250:C523–C533.
Tamai, I., Senmaru, M., Terasaki, T., and Tsuji, A. 1995. Na+-and Cl−-dependent transport of taurine at the blood-brain barrier. Biochem. Pharmacol. 50:1783–1793.
Uchida, S., Kwon, H. M., Preston, A. S., and Handler, J. S. 1991. Expression of Madin-Darby canine kidney cell Na+-and Cl+-dependent taurine transporter in Xenopus laevis oocytes. J. Biol. Chem. 266:9605–9609.
Lambert, I. H., Hoffmann, E. K., and Jorgensen, F. 1989. Membrane potential, anion and cation conductances in Ehrlich ascites tumor cells. J. Membr. Biol. 111:113–131.
Cabantchik, Z. I. and Greger, R. 1992. Chemical probes for anion transporters of mammalian cell membranes. Am. J. Physiol. 262: C803–C827.
Zelikovic, I. and Chesney, R. W. 1989. Ionic requirements for amino acid transport. Am. J. Kidney Dis. 14:313–316.
Bogé, G., Roche, H., and Pérés, G. 1985. Role of chloride ions in glycine transport in a sea fish, the bass (Dicentrarchus labrax). Biochim. Biophys. Acta 820:122–130.
Wolff, N. A., Perlman, D. F., and Goldstein, L. 1986. Ionic requirements of peritubular taurine transport in Fundulus kidney. Am. J. Physiol. 250:R984–R990.
Loo, D. D., Eskandari, S., Boorer, K. J., Sarkar, H. K., and Wright, E. M. 2000. Role of Cl− in electrogenic Na+-coupled cotransporters GAT1 and SGLT1. J. Biol. Chem. 275:37414–37422.
Lambert, I. H. and Hoffmann, E. K. 1993. Regulation of taurine transport in Ehrlich ascites tumor cells. J. Membr. Biol. 131:67–79.
Barnard, J. A., Thaxter, S., Kikuchi, K., and Ghishan, F. K. 1988. Taurine transport by rat intestine. Am. J. Physiol. 254:G334–G338.
Liu, Q. R., Lopez-Corcuera, B., Nelson, H., Mandiyan, S., and Nelson, N. 1992. Cloning and expression of a cDNA encoding the transporter of taurine and beta-alanine in mouse brain. Proc. Natl. Acad. Sci. USA 89:12145–12149.
Loo, D. D., Hirsch, J. R., Sarkar, H. K., and Wright, E. M. 1996. Regulation of the mouse retinal taurine transporter (TAUT) by protein kinases in Xenopus oocytes. FEBS Lett. 392:250–254.
Han, X., Budreau, A. M., and Chesney, R. W. 1996. Role of conserved peptide in taurine transporter inactivation modulated by protein kinase C. J. Am. Soc. Nephrol. 7:2088–2096.
Xu, Y. X., Wagenfeld, A., Yeung, C. H., Lehnert, W., and Cooper, T. G. 2003. Expression and location of taurine transporters and channels in the epididymis of infertile c-ros receptor tyrosine kinase-deficient and fertile heterozygous mice. Mol. Reprod. Dev. 64:144–151.
Mollerup, J. and Lambert, I. H. 1996. Phosphorylation is involved in the regulation of the taurine influx via the beta-system in Ehrlich ascites tumor cells. J. Membr. Biol. 150:73–82.
Tchoumkeu-Nzouessa, G. C. and Rebel, G. 1996. Regulation of taurine transport in rat astrocytes by protein kinase C: Role of calcium and calmodulin. Am. J. Physiol. 270:C1022–C1028.
Qian, X., Vinnakota, S., Edwards, C., and Sarkar, H. K. 2000. Molecular characterization of taurine transport in bovine aortic endothelial cells. Biochim. Biophys. Acta 1509:324–334.
Han, X., Budreau, A. M., and Chesney, R. W. 1999. Ser-322 is a critical site for PKC regulation of the MDCK cell taurine transporter (pNCT). J. Am. Soc. Nephrol. 10:1874–1879.
Huxtable, R. J., Chubb, J., and Azari, J. 1980. Physiological and experimental regulation of taurine content in the heart. Fed. Proc. 39:2685–2690.
Larsen, A. K., Jensen, B. S., and Hoffmann, E. K. 1994. Activation of protein kinase C during cell volume regulation in Ehrlich mouse ascites tumor cells. Biochim. Biophys. Acta 1222:477–482.
Sanchez-Olea, R., Moran, J., and Pasantes-Morales, H. 1992. Changes in taurine transport evoked by hyperosmolarity in cultured astrocytes. J. Neurosci. Res. 32:86–92.
Shimizu, M. and Satsu, H. 2000. Physiological significance of taurine and the taurine transporter in intestinal epithelial cells. Amino Acids 19:605–614.
Miyakawa, H., Woo, S. K., Dahl, S. C., Handler, J. S., and Kwon, H. M. 1999. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc. Natl. Acad. Sci. USA 96:2538–2542.
Woo, S. K., Dahl, S. C., Handler, J. S., and Kwon, H. M. 2000. Bidirectional regulation of tonicity-responsive enhancer binding protein in response to changes in tonicity. Am. J. Physiol. Renal Physiol. 278:F1006–F1012.
Nadkarni, V., Gabbay, K. H., Bohren, K. M., and Sheikh-Hamad, D. 1999. Osmotic response element enhancer activity: Regulation through p38 kinase and mitogen-activated extracellular signal-regulated kinase kinase. J. Biol. Chem. 274:20185–20190.
Dahl, S. C., Handler, J. S., and Kwon, H. M. 2001. Hypertonicity-induced phosphorylation and nuclear localization of the transcription factor TonEBP. Am. J. Physiol. Cell Physiol. 280:C248–C253.
Han, X., Chesney, R. W., Budreau, A. M., and Jones, D. P. 1996. Regulation of expression of taurine transport in two continous renal epithelial cell lines and inhibition of taurine transporter by a site-directed antibody. Adv. Exp. Med. Biol. 403:173–191.
Bitoun, M. and Tappaz, M. 2000. Taurine down-regulates basal and osmolarity-induced gene expression of its transporter, but not the gene expression of its biosynthetic enzymes, in astrocyte primary cultures. J. Neurochem. 75:919–924.
Han, X., Budreau, A. M., and Chesney, R. W. 2000. Cloning and characterization of the promoter region of the rat taurine transporter (TauT) gene. Adv. Exp. Med. Biol. 483:97–108.
Saransaari, P. and Oja, S. S. 1998. Mechanisms of ischemia-induced taurine release in mouse hippocampal slices. Brain Res. 807:118–124.
Pasantes-Morales, H., Moran, J., and Sanchez-Olea, R. 1992. Volume regulatory fluxes in glial and renal cells. Adv. Exp. Med. Biol. 315:361–368.
Ruhfus, B. and Kinne, R. K. 1996. Hypotonicity-activated efflux of taurine and myo-inositol in rat inner medullary collecting duct cells: Evidence for a major common pathway. Kidney Blood Press. Res. 19:317–324.
Lambert, I. H. 2003. Reactive oxygen species regulate swelling-induced taurine efflux in NIH3T3 mouse fibroblasts. J. Membr. Biol. 192:19–32.
Hoffmann, E. K., Lambert, I. H., and Simonsen, L. O. 1986. Separate, Ca2+-activated K+ and Cl− transport pathways in Ehrlich ascites tumor cells. J. Membr. Biol. 91:227–244.
Hoffmann, E. K. and Hendil, K. B. 1976. The role of amino acids and taurine in isosmotic intracellular regulation in Ehrlich ascites mouse tumour cells. J. Comp. Physiol. 108:279–286.
Law, R. O. 1991. Amino acids as volume-regulatory osmolytes in mammalian cells. Comp. Biochem. Physiol. A 99:263–277.
Lambert, I. H. and Hoffmann, E. K. 1982. Amino acid metabolism and protein turnover under different osmotic conditions in Ehrlich ascites tumor cells. Mol. Physiol. 2:273–286.
Margalit, A., Livne, A. A., Funder, J., and Granot, Y. 1993. Initiation of RVD response in human platelets: Mechanical-biochemical transduction involves pertussis-toxin-sensitive G protein and phospholipase A2. J. Membr. Biol. 136:303–311.
Basavappa, S., Pedersen, S. F., Jørgensen, N. K., Ellory, J. C., and Hoffmann, E. K. 1998. Swelling-induced arachidonic acid release via the 85-kDa cPLA2 in human neuroblastoma cells. J. Neurophysiol. 79:1441–1449.
Lambert, I. H. and Sepulveda, F. V. 2000. Swelling-induced taurine efflux from HeLa cells: Cell volume regulation. Adv. Exp. Med. Biol. 483:487–495.
Kudo, I. and Murakami, M. 2002. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 68-69:3–58.
Hirabayashi, T. and Shimizu, T. 2000. Localization and regulation of cytosolic phospholipase A2. Biochim. Biophys. Acta 1488: 124–138.
Winstead, M. V., Balsinde, J., and Dennis, E. A. 2000. Calcium-independent phospholipase A2: Structure and function. Biochim. Biophys. Acta 1488:28–39.
Hanel, A. M., Schuttel, S., and Gelb, M. H. 1993. Processive interfacial catalysis by mammalian 85-kilodalton phospholipase A2 enzymes on product-containing vesicles: Application to the determination of substrate preferences. Biochemistry 32:5949–5958.
Atsumi, G., Murakami, M., Kojima, K., Hadano, A., Tajima, M., and Kudo, I. 2000. Distinct roles of two intracellular phospholipase A2s in fatty acid release in the cell death pathway: Proteolytic fragment of type IVA cytosolic phospholipase A2α inhibits stimulus-induced arachidonate release, whereas that of type VI Ca2+-independent phospholipase A2 augments spontaneous fatty acid release. J. Biol. Chem. 275:18248–18258.
Martinez, J. and Moreno, J. J. 2001. Role of Ca2+-independent phospholipase A2 on arachidonic acid release induced by reactive oxygen species. Arch. Biochem. Biophys. 392:257–262.
Henderson, L. M., Chappell, J. B., and Jones, O. T. 1989. Superoxide generation is inhibited by phospholipase A2 inhibitors: Role for phospholipase A2 in the activation of the NADPH oxidase. Biochem. J. 264:249–255.
Street, I. P., Lin, H. K., Laliberte, F., Ghomashchi, F., Wang, Z., Perrier, H., Tremblay, N. M., Huang, Z., Weech, P. K., and Gelb, M. H: 1993. Slow-and tight-binding inhibitors of the 85-kDa human phospholipase A2. Biochemistry 32:5935–5940.
Thoroed, S. M., Lauritzen, L., Lambert, I. H., Hansen, H. S., and Hoffmann, E. K. 1997. Cell swelling activates phospholipase A2 in Ehrlich ascites tumor cells. J. Membr. Biol. 160:47–58.
Tinel, H., Kinne-Saffran, E., and Kinne, R. K. 2000. Calcium signalling during RVD of kidney cells. Cell Physiol. Biochem. 10: 297–302.
Pedersen, S., Lambert, I. H., Thoroed, S. M., and Hoffmann, E. K. 2000. Hypotonic cell swelling induces translocation of the alpha isoform of cytosolic phospholipase A2 but not the gamma isoform in Ehrlich ascites tumor cells. Eur. J. Biochem. 267: 5531–5539.
Sierra-Honigmann, M. R., Bradley, J. R., and Pober, J. S. 1996. Cytosolic phospholipase A2 is in the nucleus of subconfluent endothelial cells but confined to the cytoplasm of confluent endothelial cells and redistributes to the nuclear envelope and cell junctions upon histamine stimulation. Lab. Invest. 74:684–695.
Pedersen, S. F., Hoffmann, E. K., and Mills, J. W. 2001. The cytoskeleton and cell volume regulation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 130:385–399.
Peters-Golden, M., Song, K., Marshall, T., and Brock, T. 1996. Translocation of cytosolic phospholipase A2 to the nuclear envelope elicits topographically localized phospholipid hydrolysis. Biochem. J. 318:797–803.
Evans, J. H., Spencer, D. M., Zweifach, A., and Leslie, C. C. 2001. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes. J. Biol. Chem. 276: 30150–30160.
Jørgensen, N. K., Christensen, S., Harbak, H., Brown, A. M., Lambert, I. H., Hoffmann, E. K., and Simonsen, L. O. 1997. On the role of calcium in the regulatory volume decrease (RVD) response in Ehrlich mouse ascites tumor cells. J. Membr. Biol. 157:281–299.
Estevez, A. Y., O'Regan, M. H., Song, D., and Phillis, J. W. 1999. Hyposmotically induced amino acid release from the rat cerebral cortex: Role of phospholipases and protein kinases. Brain Res. 844:1–9.
Claesson, H. E. and Dahlen, S. E. 1999. Asthma and leukotrienes: Antileukotrienes as novel anti-asthmatic drugs. J. Intern. Med. 245:205–227.
Ordway, R. W., Singer, J. J., and Walsh, J. V., Jr. 1991. Direct regulation of ion channels by fatty acids. Trends Neurosci. 14: 96–100.
Maingret, F., Patel, A. J., Lesage, F., Lazdunski, M., and Honore, E. 2000. Lysophospholipids open the two-pore domain mechanogated K+ channels TREK-1 and TRAAK. J. Biol. Chem. 275: 10128–10133.
Lambert, I. H. 1991. Effect of arachidonic acid on conductive Na, K and anion transport in Ehrlich ascites tumor cells under isotonic and hypotonic conditions. Cell. Physiol. Biochem. 1: 177–194.
Lambert, I. H. 1998. Regulation of the taurine content in Ehrlich ascites tumour cells. Adv. Exp. Med. Biol. 442:269–276.
Ruhfus, B., Tinel, H., and Kinne, R. K. 1996. Role of G-proteins in the regulation of organic osmolyte efflux from isolated rat renal inner medullary collecting duct cells. Pflugers Arch. 433:35–41.
Lambert, I. H. 1987. Effect of arachidonic acid, fatty acids, prostaglandins, and leukotrienes on volume regulation in Ehrlich ascites tumor cells. J. Membr. Biol. 98:207–221.
Sanchez-Olea, R., Morales-Mulia, M., Moran, J., and Pasantes-Morales, H. 1995. Inhibition by polyunsaturated fatty acids of cell volume regulation and osmolyte fluxes in astrocytes. Am. J. Physiol. 269:C96–102.
Hall, J. A., Kirk, J., Potts, J. R., Rae, C., and Kirk, K. 1996. Anion channel blockers inhibit swelling-activated anion, cation, and nonelectrolyte transport in HeLa cells. Am. J. Physiol. 271: C579–C588.
Rådmark, O. P. 2000. The molecular biology and regulation of 5-lipoxygenase. Am. J. Respir. Crit. Care Med. 161:S11–S15.
Nicosia, S., Capra, V., and Rovati, G. E. 2001. Leukotrienes as mediators of asthma. Pulm. Pharmacol. Ther. 14:3–19.
Spector, A. A., Gordon, J. A., and Moore, S. A. 1988. Hydroxyeicosatetraenoic acids (HETEs). Prog. Lipid Res. 27:271–323.
Brash, A. R. 1999. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 274:23679–23682.
Rouzer, C. A., Ford-Hutchinson, A. W., Morton, H. E., and Gillard, J. W. 1990. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J. Biol. Chem. 265:1436–1442.
Noguchi, M., Miyano, M., and Matsumoto, T. 1996. Physiochemical characterization of ATP binding to human 5-lipoxygenase. Lipids 31:367–371.
Werz, O., Burkert, E., Fischer, L., Szellas, D., Dishart, D., Samuelsson, B., Radmark, O., and Steinhilber, D. 2002. Extracellular signal-regulated kinases phosphorylate 5-lipoxygenase and stimulate 5-lipoxygenase product formation in leukocytes. FASEB J. 16:1441–1443.
Lepley, R. A., Muskardin, D. T., and Fitzpatrick, F. A. 1996. Tyrosine kinase activity modulates catalysis and translocation of cellular 5-lipoxygenase. J. Biol. Chem. 271:6179–6184.
Mastrocola, T., Lambert, I. H., Kramhøft, B., Rugolo, M., and Hoffmann, E. K. 1993. Volume regulation in human fibroblasts: Role of Ca2+ and 5-lipoxygenase products in the activation of the Cl− efflux. J. Membr. Biol. 136:55–62.
Lambert, I. H., Nielsen, J. H., Andersen, H. J., and ørtenblad, N. 2001. Cellular model for induction of drip loss in meat. J. Agric. Food Chem. 49:4876–4883.
ørtenblad, N., Young, J. F., Oksbjerg, N., Nielsen, J. H., and Lambert, I. H. 2003. Reactive oxygen species are important mediators of taurine release from skeletal muscle cells. Am. J. Physiol. 284:C1362–C1373.
Furlong, T. J., Moriyama, T., and Spring, K. R. 1991. Activation of osmolyte efflux from cultured renal papillary epithelial cells. J. Membr. Biol. 123:269–277.
Margalit, A., Sofer, Y., Grossman, S., Reynaud, D., Pace-Asciak, C. R., and Livne, A. A. 1993. Hepoxilin A3 is the endogenous lipid mediator opposing hypotonic swelling of intact human platelets. Proc. Natl. Acad. Sci. USA 90:2589–2592.
Lambert, I. H. 1994. Eicosanoids and cell volume regulation. Pages 279–298, in Strange, K.(ed.), Cellular and molecular physiology of cell volume regulation. Boca Raton, Fla: CRC Press.
Lambert, I. H., Hoffmann, E. K., and Christensen, P. 1987. Role of prostaglandins and leukotrienes in volume regulation by Ehrlich ascites tumor cells. J. Membr. Biol. 98:247–256.
Lauritzen, L., Hoffmann, E. K., Hansen, H. S., and Jensen, B. 1993. Dietary n-3 and n-6 fatty acids are equipotent in stimulating volume regulation in Ehrlich ascites tumor cells. Am. J. Physiol. 264:C109–C117.
Lambert, I. H. 1989. Leukotriene-D4 induced cell shrinkage in Ehrlich ascites tumor cells. J. Membr. Biol. 108:165–176.
Jørgensen, N. K., Lambert, I. H., and Hoffmann, E. K. 1996. Role of LTD4 in the regulatory volume decrease response in Ehrlich ascites tumor cells. J. Membr. Biol. 151:159–173.
Hoffmann, E. K. 1999. Leukotriene D4 (LTD4) activates charybdotoxin-sensitive and-insensitive K+ channels in Ehrlich ascites tumor cells. Pflugers Arch. 438:263–268.
Hougaard, C., Niemeyer, M. I., Hoffmann, E. K., and Sepulveda, F. V. 2000. K+ currents activated by leukotriene D4 or osmotic swelling in Ehrlich ascites tumour cells. Pflugers Arch. 440: 283–294.
Strange, K., Morrison, R., Shrode, L., and Putnam, R. 1993. Mechanism and regulation of swelling-activated inositol efflux in brain glial cells. Am. J. Physiol. 265:C244–C256.
Diener, M. and Scharrer, E. 1993. The leukotriene D4 receptor blocker, SK&F 104353, inhibits volume regulation in isolated crypts from the rat distal colon. Eur. J. Pharmacol. 238:217–222.
Mignen, O., Le Gall, C., Harvey, B. J., and Thomas, S. 1999. Volume regulation following hypotonic shock in isolated crypts of mouse distal colon. J. Physiol. 515:501–510.
O'Connor, E. R. and Kimelberg, H. K. 1993. Role of calcium in astrocyte volume regulation and in the release of ions and amino acids. J. Neurosci. 13:2638–2650.
Shen, M. R., Chou, C. Y., Browning, J. A., Wilkins, R. J., and Ellory, J. C. 2001. Human cervical cancer cells use Ca2+ signalling, protein tyrosine phosphorylation and MAP kinase in regulatory volume decrease. J. Physiol. 537:347–362.
Foskett, J. K., Wong, M. M., Sue, A. Quan, and Robertson, M. A. 1994. Isosmotic modulation of cell volume and intracellular ion activities during stimulation of single exocrine cells. J. Exp. Zool. 268:104–110.
Morales, M. S., Vaca, L., Hernandez, C. A., and Pasantes, M. H. 1998. Osmotic swelling-induced changes in cytosolic calcium do not affect regulatory volume decrease in rat cultured suspended cerebellar astrocytes. J. Neurochem. 71:2330–2338.
Grinstein, S. and Smith, J. D. 1990. Calcium-independent cell volume regulation in human lymphocytes: Inhibition by charybdotoxin. J. Gen. Physiol. 95:97–120.
Vitarella, D., DiRisio, D. J., Kimelberg, H. K., and Aschner, M. 1994. Potassium and taurine release are highly correlated with regulatory volume decrease in neonatal primary rat astrocyte cultures. J. Neurochem. 63:1143–1149.
Lang, F., Madlung, J., Siemen, D., Ellory, C., Lepple-Wienhues, A., and Gulbins, E. 2000. The involvement of caspases in the CD95 (Fas/Apo-1)-but not swelling-induced cellular taurine release from Jurkat T-lymphocytes. Pflugers Arch. 440:93–99.
Moran, J., Morales, M. S., Hernandez, C. A., and Pasantes, M. H. 1997. Regulatory volume decrease and associated osmolyte fluxes in cerebellar granule neurons are calcium independent. J. Neurosci. Res. 47:144–154.
Deleuze, C., Duvoid, A., and Hussy, N. 1998. Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. J. Physiol. 507:463–471.
Katz, U., Lancaster, J. A., and Ellory, J. C. 2003. Hypotonic-induced transport pathways in Xenopus laevis erythrocytes: Taurine fluxes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 134:355–363.
Pierce, S. K. and Rowland-Faux, L. M. 1992. Ionomycin produces an improved volume recovery by an increased efflux of taurine from hypoosmotically stressed molluscan red blood cells. Cell Calcium 13:321–327.
Galietta, L. J., Falzoni, S., Di Virgilio, F., Romeo, G., and Zegarra-Moran, O. 1997. Characterization of volume-sensitive taurine-and Cl−-permeable channels. Am. J. Physiol. 273: C57–C66.
Pedersen, S., Hoffmann, E. K., Hougaard, C., Jørgensen, N. K., Wybrandt, G. B., and Lambert, I. H. 1997. Leukotriene D4induced Ca2+ mobilization in Ehrlich ascites tumor cells. J. Membr. Biol. 155:61–73.
Sjølander, A., Gronroos, E., Hammarstrom, S., and Andersson, T. 1990. Leukotriene D4 and E4 induce transmembrane signaling in human epithelial cells: Single cell analysis reveals diverse pathways at the G-protein level for the influx and the intracellular mobilization of Ca2+. J. Biol. Chem. 265:20976–20981.
Adolfsson, J. L., Ohd, J. F., and Sjølander, A. 1996. Leukotriene D4-induced activation and translocation of the G-protein alpha i3-subunit in human epithelial cells. Biochem. Biophys. Res. Commun. 226:413–419.
Grønroos, E., Andersson, T., Schippert, A., Zheng, L., and Sjølander, A. 1996. Leukotriene D4-induced mobilization of intracellular Ca2+ in epithelial cells is critically dependent on activation of the small GTP-binding protein Rho. Biochem. J. 316:239–245.
Thodeti, C. K., Massoumi, R., Bindslev, L., and Sjolander, A. 2002. Leukotriene D4 induces association of active RhoA with phospholipase C-gammal in intestinal epithelial cells. Biochem. J. 365:157–163.
Kirk, J. and Kirk, K. 1994. Inhibition of volume-activated I-and taurine efflux from HeLa cells by P-glycoprotein blockers correlates with calmodulin inhibition. J. Biol. Chem. 269:29389–29394.
Huang, C. C., Chang, C. B., Liu, J. Y., Basavappa, S., and Lim, P. H. 2001. Effects of calcium, calmodulin, protein kinase C and protein tyrosine kinases on volume-activated taurine efflux in human erythroleukemia cells. J. Cell Physiol. 189:316–322.
Li, G., Liu, Y., and Olson, J. E. 2002. Calcium/calmodulin-modulated chloride and taurine conductances in cultured rat astrocytes. Brain Res. 925:1–8.
Jenkins, C. M., Wolf, M. J., Mancuso, D. J., and Gross, R. W. 2001. Identification of the calmodulin-binding domain of recombinant calcium-independent phospholipase A2beta: Implications for structure and function. J. Biol. Chem. 276:7129–7135.
Nielsen, C. K. and Lambert, I. H. 1998. Characterisation of the leukotriene D4 receptor in the Ehrlich ascites tumour cells. Acta Physiol. Scand. 163:P15.
Lambert, I. H. and Falktoft, B. 2001. Lysophosphatidylcholine-induced taurine release in HeLa cells involves protein kinase activity. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 130:577–584.
Tilly, B. C., van den Berghe, N., Tertoolen, L. G., Edixhoven, M. J., and de Jonge, H. R. 1993. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. J. Biol. Chem. 268:19919–19922.
Tilly, B. C., Edixhoven, M. J., van den, Berghe N., Bot, A. G., and de Jonge, H. R. 1994. Ca2+-Mobilizing hormones potentiate hypotonicity-induced activation of ionic conductances in Intestine 407 cells. Am. J. Physiol. 267:C1271–C1278.
Nilius, B. and Droogmans, G. 2003. Amazing chloride channels: An overview. Acta Physiol. Scand. 177:119–147.
Ballatori, N. and Boyer, J. L. 1997. ATP regulation of a swelling-activated osmolyte channel in skate hepatocytes. J. Exp. Zool. 279:471–475.
Ballatori, N. and Wang, W. 1997. Nordihydroguaiaretic acid depletes ATP and inhibits a swelling-activated, ATP-sensitive taurine channel. Am. J. Physiol. 272:C1429–C1436.
Wang, Y., Roman, R., Lidofsky, S. D., and Fitz, J. G. 1996. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc. Natl. Acad. Sci. USA 93:12020–12025.
Okada, Y., Maeno, E., Shimizu, T., Dezaki, K., Wang, J., and Morishima, S. 2001. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J. Physiol. 532:3–16.
Junankar, P. R., Karjalainen, A., and Kirk, K. 2002. The role of P2Y1 purinergic receptors and cytosolic Ca2+ in hypotonically activated osmolyte efflux from a rat hepatoma cell line. J. Biol. Chem. 277:40324–40334.
Dutta, A. K., Okada, Y., and Sabirov, R. Z. 2002. Regulation of an ATP-conductive large-conductance anion channel and swelling-induced ATP release by arachidonic acid. J. Physiol. 542:803–816.
Braunstein, G. M., Roman, R. M., Clancy, J. P., Kudlow, B. A., Taylor, A. L., Shylonsky, V. G., Jovov, B., Peter, K., Jilling, T., Ismailov, I. I., Benos, D. J., Schwiebert, L. M., Fitz, J. G., and Schwiebert, E. M. 2001. Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J. Biol. Chem. 276:6621–6630.
Steinacker, P., Sternbach, H., Rohm, B., Walter, G., Thinnes, F. P., and Hilschmann, N. 1997. VDAC is involved in osmotically activated taurine-efflux in HeLa cells. Pflugers Arch. 433:R162.
Nilius, B., Sehrer, J., Viana, F., De Greef, C., Raeymaekers, L., Eggermont, J., and Droogmans, G. 1994. Volume-activated Cl− currents in different mammalian non-excitable cell types. Pflugers Arch. 428:364–371.
Jackson, P. S. and Strange, K. 1995. Single-channel properties of a volume-sensitive anion conductance: Current activation occurs by abrupt switching of closed channels to an open state. J. Gen. Physiol. 105:643–660.
Ralevic, V. and Burnstock, G. 1998. Receptors for purines and pyrimidines. Pharmacol. Rev. 50:413–492.
van der Weyden, L., Adams, D. J., Luttrell, B. M., Conigrave, A. D., and Morris, M. B. 2000. Pharmacological characterisation of the P2Y11 receptor in stably transfected haematological cell lines. Mol. Cell Biochem. 213:75–81.
Fredholm, B. B., Abbracchio, M. P., Burnstock, G., Dubyak, G. R., Harden, T. K., Jacobson, K. A., Schwabe, U., and Williams, M. 1997. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol. Sci. 18:79–82.
Pedersen, S. F., Pedersen, S., Lambert, I. H., and Hoffmann, E. K. 1998. P2 receptor-mediated signal transduction in Ehrlich ascites tumor cells. Biochim. Biophys. Acta 1374:94–106.
Pedersen, S., Pedersen, S. F., Nilius, B., Lambert, I. H., and Hoffmann, E. K. 1999. Mechanical stress induces release of ATP from Ehrlich ascites tumor cells. Biochim. Biophys. Acta 1416: 271–284.
Zimmermann, H. 2000. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedebergs Arch. Pharmacol. 362:299–309.
Thannickal, V. J. and Fanburg, B. L. 2000. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L1005–L1028.
Finkel, T. 2001. Reactive oxygen species and signal transduction. IUBMB Life 52:3–6.
Kugiyama, K., Sugiyama, S., Ogata, N., Oka, H., Doi, H., Ota, Y., and Yasue, H. 1999. Burst production of superoxide anion in human endothelial cells by lysophosphatidylcholine. Atherosclerosis 143:201–204.
Yamakawa, T., Tanaka, S., Yamakawa, Y., Kamei, J., Numaguchi, K., Motley, E. D., Inagami, T., and Eguchi, S. 2002. Lysophosphatidylcholine activates extracellular signal-regulated kinases 1/2 through reactive oxygen species in rat vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 22: 752–758.
Chahine, R. and Feng, J. 1998. Protective effects of taurine against reperfusion-induced arrhythmias in isolated ischemic rat heart. Arzneimittelforschung 48:360–364.
Raschke, P., Massoudy, P., and Becker, B. F. 1995. Taurine protects the heart from neutrophil-induced reperfusion injury. Free Radic. Biol. Med. 19:461–471.
Demmig-Adams, B. and Adams, W. W., III. 2002. Antioxidants in photosynthesis and human nutrition. Science 298:2149–2153.
Filomeni, G., Rotilio, G., and Ciriolo, M. R. 2002. Cell signalling and the glutathione redox system. Biochem. Pharmacol. 64:1057–1064.
Vepa, S., Scribner, W. M., Parinandi, N. L., English, D., Garcia, J. G., and Natarajan, V. 1999. Hydrogen peroxide stimulates tyrosine phosphorylation of focal adhesion kinase in vascular endothelial cells. Am. J. Physiol. 277:L150–L158.
Pedersen, S. F., Beisner, K. H., Hougaard, C., Willumsen, B. M., Lambert, I. H., and Hoffmann, E. K. 2002. Rho family GTP binding proteins are involved in the regulatory volume decrease process in NIH3T3 mouse fibroblasts. J. Physiol. 541:779–796.
Lambert, I. H. 2003. Regulation of the volume-sensitive taurine efflux pathway in NIH3T3 mouse fibroblasts. In Azuma, J., Lombardini, J. B., Schaffer, S. W., and Azuma, J. Taurine in the 21st century, (in press).
Deleuze, C., Duvoid, A., Moos, F. C., and Hussy, N. 2000. Tyrosine phosphorylation modulates the osmosensitivity of volume-dependent taurine efflux from glial cells in the rat supraoptic nucleus. J. Physiol. 523:291–299.
Lambert, I. H. and Falktoft, B. 2000. Lysophosphatidylcholine induces taurine release from HeLa cells. J. Membr. Biol. 176: 175–185.
Meng, T. C., Fukada, T., and Tonks, N. K. 2002. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 9:387–399.
Kamata, H. and Hirata, H. 1999. Redox regulation of cellular signalling. Cell. Signal. 11:1–14.
Lepple-Wienhues, A., Szabo, I., Laun, T., Kaba, N. K., Gulbins, E., and Lang, F. 1998. The tyrosine kinase p56lck mediates activation of swelling-induced chloride channels in lymphocytes. J. Cell Biol. 141:281–286.
Ochoa de la Paz, L. D., Lezama, R., Torres-Marquez, M. E., and Pasantes-Morales, H. 2002. Tyrosine kinases and amino acid efflux under hyposmotic and ischaemic conditions in the chicken retina. Pflugers Arch. 445:87–96.
Mongin, A. A., Reddi, J. M., Charniga, C., and Kimelberg, H. K. 1999. [3H]taurine and D-[3H]aspartate release from astrocyte cultures are differently regulated by tyrosine kinases. Am. J. Physiol. 276:C1226–C1230.
Hubert, E. M., Musch, M. W., and Goldstein, L. 2000. Inhibition of volume-stimulated taurine efflux and tyrosine kinase activity in the skate red blood cell. Pflugers Arch. 440: 132–139.
Shaul, P. W. and Anderson, R. G. 1998. Role of plasmalemmal caveolae in signal transduction. Am. J. Physiol. 275:L843–L851.
Trouet, D., Carton, I., Hermans, D., Droogmans, G., Nilius, B., and Eggermont, J. 2001. Inhibition of VRAC by c-Src tyrosine kinase targeted to caveolae is mediated by the Src homology domains. Am. J. Physiol. Cell Physiol. 281:C248–C256.
Davis, M. J., Wu, X., Nurkiewicz, T. R., Kawasaki, J., Gui, P., Hill, M. A., and Wilson, E. 2001. Regulation of ion channels by protein tyrosine phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 281:H1835–H1862.
Tilly, B. C., Edixhoven, M. J., Tertoolen, L. G., Morii, N., Saitoh, Y., Narumiya, S., and de Jonge, H. R. 1996. Activation of the osmo-sensitive chloride conductance involves P21rho and is accompanied by a transient reorganization of the F-actin cytoskeleton. Mol. Biol. Cell 7:1419–1427.
Ben Mahdi, M. H., Andrieu, V., and Pasquier, C. 2000. Focal adhesion kinase regulation by oxidative stress in different cell types. IUBMB Life 50:291–299.
Salazar, E. P. and Rozengurt, E. 1999. Bombesin and platelet-derived growth factor induce association of endogenous focal adhesion kinase with Src in intact Swiss 3T3 cells. J. Biol. Chem. 274:28371–28378.
Maa, M. C. and Leu, T. H. 1998. Vanadate-dependent FAK activation is accomplished by the sustained FAK Tyr-576/577 phosphorylation. Biochem. Biophys. Res. Commun. 251:344–349.
Barchowsky, A., Munro, S. R., Morana, S. J., Vincenti, M. P., and Treadwell, M. 1995. Oxidant-sensitive and phosphorylation-dependent activation of NF-kappa B and AP-1 in endothelial cells. Am. J. Physiol. 269:L829–L836.
Widmann, C., Gibson, S., Jarpe, M. B., and Johnson, G. L. 1999. Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol. Rev. 79:143–180.
Shen, M. R., Chou, C. Y., Browning, J. A., Wilkins, R. J., and Ellory, J. C. 2001. Human cervical cancer cells use Ca2+ signalling, protein tyrosine phosphorylation and MAP kinase in regulatory volume decrease. J. Physiol 537:347–362.
Haimovich, B., Ji, P., Ginalis, E., Kramer, R., and Greco, R. 1999. Phospholipase A2 enzymes regulate αIIb β3-mediated, but not Fc γRII receptor-mediated, pp125FAK phosphorylation in platelets. Thromb. Haemost. 81:618–624.
Voets, T., Manolopoulos, V., Eggermont, J., Ellory, C., Droogmans, G., and Nilius, B. 1998. Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins. J. Physiol. 506:341–352.
Chikumi, H., Fukuhara, S., and Gutkind, J. S. 2002. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: Evidence of a role for focal adhesion kinase. J. Biol. Chem. 277: 12463–12473.
Hoffmann, E. K. and Mills, J. W. 1999. Membrane events involved in volume regulation. Curr. Topics Membr. 48:123–196.
Finkel, T. 2000. Redox-dependent signal transduction. FEBS Lett. 476:52–54.
McPhail, L. C., Waite, K. A., Regier, D. S., Nixon, J. B., Qualliotine-Mann, D., Zhang, W. X., Wallin, R., and Sergeant, S. 1999. A novel protein kinase target for the lipid second messenger phosphatidic acid. Biochim. Biophys. Acta 1439:277–290.
Babior, B. M. 1998. NADPH oxidase: An update. Blood 93: 1464–1476.
Zhao, X., Bey, E. A., Wientjes, F. B., and Cathcart, M. K. 2002. Cytosolic phospholipase A2 (cPLA2) regulation of human monocyte NADPH oxidase activity: cPLA2 affects translocation but not phosphorylation of p67(phox) and p47(phox). J. Biol. Chem. 277:25385–25392.
Javesghani, D., Magder, S. A., Barreiro, E., Quinn, M. T., and Hussain, S. N. 2002. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am. J. Respir. Crit. Care Med. 165:412–418.
Regier, D. S., Greene, D. G., Sergeant, S., Jesaitis, A. J., and McPhail, L. C. 2000. Phosphorylation of p22phox is mediated by phospholipase D-dependent and-independent mechanisms: Correlation of NADPH oxidase activity and p22phox phosphorylation. J. Biol. Chem. 275:28406–28412.
Fontayne, A., Dang, P. M., Gougerot-Pocidalo, M. A., and El Benna, J. 2002. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: Effect on binding to p22phox and on NADPH oxidase activation. Biochemistry 41:7743–7750.
Gopalakrishna, R. and Anderson, W. B. 1991. Reversible oxidative activation and inactivation of protein kinase C by the mitogen/tumor promoter periodate. Arch. Biochem. Biophys. 285: 382–387.
Konishi, H., Tanaka, M., Takemura, Y., Matsuzaki, H., Ono, Y., Kikkawa, U., and Nishizuka, Y. 1997. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc. Natl. Acad. Sci. USA 94:11233–11237.
Fang, X., Gaudette, D., Furui, T., Mao, M., Estrella, V., Eder, A., Pustilnik, T., Sasagawa, T., LaPushin, R., Y, S., Jaffe, R. B., Wiener, J. R., Erickson, J. R., and Mills, G. B. 2000. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Ann. NY Acad. Sci. 905: 188–208.
Kabarowski, J. H., Zhu, K., Le, L. Q., Witte, O. N., and Xu, Y. 2001. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science 293:702–705.
Lundbaek, J. A. and Andersen, O. S. 1994. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J. Gen. Physiol. 104:645–673.
Chen, M., Xiao, C. Y., Hashizume, H., and Abiko, Y. 1997. Differential effects of Ca2+ channel blockers on Ca2+ overload induced by lysophosphatidylcholine in cardiomyocytes. Eur. J. Pharmacol. 333:261–268.
Takahashi, K., Ohyabu, Y., Schaffer, S. W., and Azuma, J. 2000. Taurine prevents ischemia damage in cultured neonatal rat cardiomyocytes. Adv. Exp. Med. Biol. 483:109–116.
Shaikh, N. A. and Downar, E. 1981. Time course of changes in porcine myocardial phospholipid levels during ischemia: A reassessment of the lysolipid hypothesis. Circ. Res. 49:316–325.
Yu, L., Netticadan, T., Xu, Y. J., Panagia, V., and Dhalla, N. S. 1998. Mechanisms of lysophosphatidylcholine-induced increase in intracellular calcium in rat cardiomyocytes. J. Pharmacol. Exp. Ther. 286:1–8.
Sagar, P. S. and Das, U. N. 1995. Cytotoxic action of cis-unsaturated fatty acids on human cervical carcinoma (HeLa) cells in vitro. Prostaglandins Leukot. Essent. Fatty Acids 53:287–299.
Lipton, P. 1999. Ischemic cell death in brain neurons. Physiol. Rev. 79:1431–1568.
Kinnaird, A. A. A., Choy, P. C., and Man, R. Y. K. 1998. Lysophosphatidylcholine accumulation in the ischemic canine heart. Lipids 23:32–35.
Kimelberg, H. K., Goderie, S. K., Higman, S., Pang, S., and Waniewski, R. A. 1990. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J. Neurosci. 10: 1583–1591.
Schousboe, A., Sanchez-Olea, R., Moran, J., and Pasantes-Morales, H. 1991. Hyposmolarity-induced taurine release in cerebellar granule cells is associated with diffusion and not with high-affinity transport. J. Neurosci. Res. 30:661–665.
Sanchez-Olea, R., Pasantes-Morales, H., Lazaro, A., and Cereijido, M. 1991. Osmolarity-sensitive release of free amino acids from cultured kidney cells (MDCK). J. Membr. Biol. 121:1–9.
Hall, A. C. 1995. Volume-sensitive taurine transport in bovine articular chondrocytes. J. Physiol 484:755–766.
Strange, K., Emma, F., and Jackson, P. S. 1996. Cellular and molecular physiology of volume-sensitive anion channels. Am. J. Physiol. 270:C711–C730.
Kirk, K. 1997. Swelling-activated organic osmolyte channels. J. Membr. Biol. 158:1–16.
Okada, Y. 1997. Volume expansion-sensing outward-rectifier Cl− channel: Fresh start to the molecular identity and volume sensor. Am. J. Physiol. 273:C755–C789.
Ackerman, M. J., Wickman, K. D., and Clapham, D. E. 1994. Hypotonicity activates a native chloride current in Xenopus oocytes. J. Gen. Physiol. 103:153–179.
Jentsch, T. J., Stein, V., Weinreich, F., and Zdebik, A. A. 2002. Molecular structure and physiological function of chloride channels. Physiol. Rev. 82:503–568.
Jackson, P. S. and Strange, K. 1993. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am. J. Physiol. 265:C1489–C1500.
Boese, S. H., Kinne, R. K., and Wehner, F. 1996. Single-channel properties of swelling-activated anion conductance in rat inner medullary collecting duct cells. Am. J. Physiol. 271:F1224–F1233.
Banderali, U. and Roy, G. 1992. Anion channels for amino acids in MDCK cells. Am. J. Physiol. 263:C1200–C1207.
Paulmichl, M., Li, Y., Wickman, K., Ackerman, M., Peralta, E., and Clapham, D. 1992. New mammalian chloride channel identified by expression cloning. Nature 356:238–241.
Thiemann, A., Grunder, S., Pusch, M., and Jentsch, T. J. 1992. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature 356:57–60.
Duan, D., Winter, C., Cowley, S., Hume, J. R., and Horowitz, B. 1997. Molecular identification of a volume-regulated chloride channel. Nature 390:417–421.
Moorman, J. R. and Jones, L. R. 1998. Phospholemman: A cardiac taurine channel involved in regulation of cell volume. Adv. Exp. Med. Biol. 442:219–228.
Fievet, B., Gabillat, N., Borgese, F., and Motais, R. 1995. Expression of band 3 anion exchanger induces chloride current and taurine transport: Structure-function analysis. EMBO J. 14: 5158–5169.
Musch, M. W., Davis-Amaral, E. M., Vandenburgh, H. H., and Goldstein, L. 1998. Hypotonicity stimulates translocation of ICln in neonatal rat cardiac myocytes. Pflugers Arch. 436:415–422.
Nilius, B., Eggermont, J., Voets, T., Buyse, G., Manolopoulos, V., and Droogmans, G. 1997. Properties of volume-regulated anion channels in mammalian cells. Prog. Biophys. Mol. Biol. 68:69–119.
Buyse, G., Voets, T., Tytgat, J., De Greef, C., Droogmans, G., Nilius, B., and Eggermont, J. 1997. Expression of human pICln and ClC-6 in Xenopus oocytes induces an identical endogenous chloride conductance. J. Biol. Chem. 272:3615–3621.
Voets, T., Buyse, G., Tytgat, J., Droogmans, G., Eggermont, J., and Nilius, B. 1996. The chloride current induced by expression of the protein pICln in Xenopus oocytes differs from the endogenous volume-sensitive chloride current. J. Physiol 495:441–447.
Stegen, C., Matskevich, I., Wagner, C. A., Paulmichl, M., Lang, F., and Broer, S. 2000. Swelling-induced taurine release without chloride channel activity in Xenopus laevis oocytes expressing anion channels and transporters. Biochim. Biophys. Acta 1467: 91–100.
Nehrke, K., Arreola, J., Nguyen, H. V., Pilato, J., Richardson, L., Okunade, G., Baggs, R., Shull, G. E., and Melvin, J. E. 2002. Loss of hyperpolarization-activated Cl− current in salivary acinar cells from Clcn2 knockout mice. J. Biol. Chem. 277: 23604–23611.
Xiong, H., Li, C., Garami, E., Wang, Y., Ramjeesingh, M., Galley, K., and Bear, C. E. 1999. ClC-2 activation modulates regulatory volume decrease. J. Membr. Biol. 167:215–221.
Hermoso, M., Satterwhite, C. M., Andrade, Y. N., Hidalgo, J., Wilson, S. M., Horowitz, B., and Hume, J. R. 2002. ClC-3 is a fundamental molecular component of volume-sensitive outwardly rectifying Cl− channels and volume regulation in HeLa cells and Xenopus laevis oocytes. J. Biol. Chem. 277:40066–40074.
Arreola, J., Begenisich, T., Nehrke, K., Nguyen, H. V., Park, K., Richardson, L., Yang, B., Schutte, B. C., Lamb, F. S., and Melvin, J. E. 2002. Secretion and cell volume regulation by salivary acinar cells from mice lacking expression of the Clcn3 Cl− channel gene. J. Physiol 545:207–216.
Moran, J., Morales-Mulia, M., and Pasantes-Morales, H. 2001. Reduction of phospholemman expression decreases osmosensitive taurine efflux in astrocytes. Biochim. Biophys. Acta 1538: 313–320.
Moorman, J. R., Ackerman, S. J., Kowdley, G. C., Griffin, M. P., Mounsey, J. P., Chen, Z., Cala, S. E., O'Brian, J. J., Szabo, G., and Jones, L. R. 1995. Unitary anion currents through phospholemmal channel molecules. Nature 377:737–740.
Morales-Mulia, M., Pasantes-Morales, H., and Moran, J. 2000. Volume sensitive efflux of taurine in HEK293 cells overexpressing phospholemman. Biochim. Biophys. Acta 1496:252–260.
Chen, Z., Jones, L. R., O'Brian, J. J., Moorman, J. R., and Cala, S. E. 1998. Structural domains in phospholemman: A possible role for the carboxyl terminus in channel inactivation. Circ. Res. 82:367–374.
Martin del Rio, R. and Solis, J. M. 1998. The anion-exchanger AE1 is a diffusion pathway for taurine transport in rat erythrocytes. Adv. Exp. Med. Biol. 442:255–260.
Pedersen, S. F., Prenen, J., Droogmans, G., Hoffmann, E. K., and Nilius, B. 1998. Separate swelling-and Ca2+-activated anion currents in Ehrlich ascites tumor cells. J. Membr. Biol. 163: 97–110.
Lambert, I. H. and Hoffmann, E. K. 1994. Cell swelling activates separate taurine and chloride channels in Ehrlich mouse ascites tumor cells. J. Membr. Biol. 142:289–298.
Diaz, M., Valverde, M. A., Higgins, C. F., Rucareanu, C., and Sepulveda, F. V. 1993. Volume-activated chloride channels in HeLa cells are blocked by verapamil and dideoxyforskolin. Pflugers Arch. 422:347–353.
Shennan, D. B. and Thomson, J. 2000. Further evidence for the existence of a volume-activated taurine efflux pathway in rat mammary tissue independent from volume-sensitive Cl− channels. Acta Physiol. Scand. 168:295–299.
Roman, R. M., Wang, Y., and Fitz, J. G. 1996. Regulation of cell volume in a human biliary cell line: Activation of K+ and Cl− currents. Am. J. Physiol. 271:G239–G248.
Pasantes-Morales, H., Quesada, O., and Moran, J. 1998. Taurine: An osmolyte in mammalian tissues. Adv. Exp. Med. Biol. 442:209–217.
Lepple-Wienhues, A., Szabo, I., Wieland, U., Heil, L., Gulbins, E., and Lang, F. 2000. Tyrosine kinases open lymphocyte chloride channels. Cell Physiol. Biochem. 10:307–312.
Stutzin, A., Torres, R., Oporto, M., Pacheco, P., Eguiguren, A. L., Cid, L. P., and Sepulveda, F. V. 1999. Separate taurine and chloride efflux pathways activated during regulatory volume decrease. Am. J. Physiol. 277:C392–C402.
Pasantes-Morales, H., Moran, J., and Schousboe, A. 1990. Volume-sensitive release of taurine from cultured astrocytes: Properties and mechanism. Glia 3:427–432.
Voets, T., Droogmans, G., and Nilius, B. 1997. Modulation of voltage-dependent properties of a swelling-activated Cl− current. J. Gen. Physiol. 110:313–325.
Nilius, B., Prenen, J., and Droogmans, G. 1998. Modulation of volume-regulated anion channels by extra-and intracellular pH. Pflugers Arch. 436:742–748.
Livne, A. and Hoffmann, E. K. 1990. Cytoplasmic acidification and activation of Na+/H+ exchange during regulatory volume decrease in Ehrlich ascites tumor cells. J. Membr. Biol. 114: 153–157.
Guizouarn, H., Motais, R., Garcia-Romeu, F., and Borgese, F. 2000. Cell volume regulation: The role of taurine loss in maintaining membrane potential and cell pH. J. Physiol. 523:147–154.
Andrew, R. D., Lobinowich, M. E., and Osehobo, E. P. 1997. Evidence against volume regulation by cortical brain cells during acute osmotic stress. Exp. Neurol. 143:300–312.
Minton, A. P. 1994. Influence of macromolecular crowding in intracellular associations: A possible role in volume regulation. Pages 181–190, in Strange, K. (ed.), Cellular and molecular physiology of cell volume regulation.Boca Raton, Fla: CRC Press.
Burg, M. B. 2000. Macromolecular crowding as a cell volume sensor. Cell Physiol. Biochem. 10:251–256.
Jackson, P. S., Churchwell, K., Ballatori, N., Boyer, J. L., and Strange, K. 1996. Swelling-activated anion conductance in skate hepatocytes: Regulation by cell Cl− and ATP. Am. J. Physiol. 270:C57–C66.
Wittels, K. A., Hubert, E. M., Musch, M. W., and Goldstein, L. 2000. Osmolyte channel regulation by ionic strength in skate RBC. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279: R69–R76.
Guizouarn, H. and Motais, R. 1999. Swelling activation of transport pathways in erythrocytes: Effects of Cl3, ionic strength, and volume changes. Am. J. Physiol. 276:C210–C220.
Voets, T., Droogmans, G., Raskin, G., Eggermont, J., and Nilius, B. 1999. Reduced intracellular ionic strength as the initial trigger for activation of endothelial volume-regulated anion channels. Proc. Natl. Acad. Sci. USA 96:5298–5303.
Hoffmann, E. K. 2000. Intracellular signalling involved in volume regulatory decrease. Cell Physiol. Biochem. 10:273–288.
Hoffmann, E. K. and Pedersen, S. F. 1998. Sensors and signal transduction in the activation of cell volume regulatory ion transport systems. Contrib. Nephrol. 123:50–78.
Treharne, K. J., Marshall, L. J., and Mehta, A. 1994. A novel chloride-dependent GTP-utilizing protein kinase in plasma membranes from human respiratory epithelium. Am. J. Physiol. 267: L592–L601.
Cornet, M., Ubl, J., and Kolb, H. A. 1993. Cytoskeleton and ion movements during volume regulation in cultured PC12 cells. J. Membr. Biol. 133:161–170.
Tilly, B. C., Edixhoven, M. J., Tertoolen, L. G., Morii, N., Saitoh, Y., Narumiya, S., and de Jonge, H. R. 1996. Activation of the osmo-sensitive chloride conductance involves P21ρ and is accompanied by a transient reorganization of the F-actin cytoskeleton. Mol. Biol. Cell 7:1419–1427.
Oike, M., Schwarz, G., Sehrer, J., Jost, M., Gerke, V., Weber, K., Droogmans, G., and Nilius, B. 1994. Cytoskeletal modulation of the response to mechanical stimulation in human vascular endothelial cells. Pflugers Arch. 428:569–576.
Ding, M., Eliasson, C., Betsholtz, C., Hamberger, A., and Pekny, M. 1998. Altered taurine release following hypotonic stress in astrocytes from mice deficient for GFAP and vimentin. Brain Res. Mol. Brain Res. 62:77–81.
Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509–514.
Ridley, A. J. and Hall, A. 1992. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70:389–399.
Massoumi, R., Larsson, C., and Sjolander, A. 2002. Leukotriene D4 induces stress-fibre formation in intestinal epithelial cells via activation of RhoA and PKCdelta. J. Cell Sci. 115:3509–3515.
Saegusa, S., Tsubone, H., and Kuwahara, M. 2001. Leukotriene D(4)-induced Rho-mediated actin reorganization in human bronchial smooth muscle cells. Eur. J. Pharmacol. 413:163–171.
Giancotti, F. G. 2003. A structural view of integrin activation and signaling. Dev. Cell 4:149–151.
Schwartz, M.A. and Shattil, S. J. 2000. Signaling networks linking integrins and rho family GTPases. Trends Biochem. Sci. 25: 388–391.
Nilius, B., Voets, T., Prenen, J., Barth, H., Aktories, K., Kaibuchi, K., Droogmans, G., and Eggermont, J. 1999. Role of Rho and Rho kinase in the activation of volume-regulated anion channels in bovine endothelial cells. J. Physiol. 516:67–74.
Michaely, P. A., Mineo, C., Ying, Y. S., and Anderson, R. G. 1999. Polarized distribution of endogenous Rac1 and RhoA at the cell surface. J. Biol. Chem. 274:21430–21436.
Okada, Y. 1999. A scaffolding for regulation of volume-sensitive Cl− channels. J. Physiol. 520:2.
Nilius, B., Prenen, J., Walsh, M. P., Carton, I., Bollen, M., Droogmans, G., and Eggermont, J. 2000. Myosin light chain phosphorylation-dependent modulation of volume-regulated anion channels in macrovascular endothelium. FEBS Lett. 466:346–350.
Miyamoto, S., Teramoto, H., Coso, O. A., Gutkind, J. S., Burbelo, P. D., Akiyama, S. K., and Yamada, K. M. 1995. Integrin function: Molecular hierarchies of cytoskeletal and signaling molecules. J. Cell Biol. 131:791–805.
Fielding, C. J. 2001. Caveolae and signaling. Curr. Opin. Lipidol. 12:281–287.
Trouet, D., Nilius, B., Jacobs, A., Remacle, C., Droogmans, G., and Eggermont, J. 1999. Caveolin-1 modulates the activity of the volume-regulated chloride channel. J. Physiol. 520:113–119.
Margalit, A. and Livne, A. A. 1992. Human platelets exposed to mechanical stresses express a potent lipoxygenase product. Thromb. Haemost. 68:589–594.
Kinnunen, P. K. 2000. Lipid bilayers as osmotic response elements. Cell Physiol. Biochem. 10:243–250.
Ingber, D. E. 1997. Tensegrity: The architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 59:575–599.
Montell, C., Birnbaumer, L., and Flockerzi, V. 2002. The TRP channels, a remarkably functional family. Cell 108:595–598.
Strotmann, R., Harteneck, C., Nunnenmacher, K., Schultz, G., and Plant, T. D. 2000. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nature Cell Biol. 2:695–702.
Wissenbach, U., Bodding, M., Freichel, M., and Flockerzi, V. 2000. Trp12, a novel Trp related protein from kidney. FEBS Lett. 485:127–134.
Nilius, B., Prenen, J., Wissenbach, U., Bodding, M., and Droogmans, G. 2001. Differential activation of the volume-sensitive cation channel TRP12 (OTRPC4) and volume-regulated anion currents in HEK-293 cells. Pflugers Arch. 443:227–233.
Olson, J. E. and Li, G. Z. 1997. Increased potassium, chloride, and taurine conductances in astrocytes during hypoosmotic swelling. Glia 20:254–261.
Han, B., Luo, G., Shi, Z. Z., Barrios, R., Atwood, D., Liu, W., Habib, G. M., Sifers, R. N., Corry, D. B., and Lieberman, M. W. 2002. Gamma-glutamyl leukotrienase, a novel endothelial membrane protein, is specifically responsible for leukotriene D4 formation in vivo. Am. J. Pathol. 161:481–490.
Hoffmann, E. K. and Hougaard, C. 2001. Intracellular signalling involved in activation of the volume-sensitive K+ current in Ehrlich ascites tumour cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 130:355–366.
Massoumi, R. and Sjolander, A. 2001. Leukotriene D4 affects localisation of vinculin in intestinal epithelial cells via distinct tyrosine kinase and protein kinase C controlled events. J. Cell Sci. 114:1925–1934.
Vegesna, R. V., Mong, S., and Crooke, S. T. 1988. Leukotriene D4-induced activation of protein kinase C in rat basophilic leukemia cells. Eur. J. Pharmacol. 147:387–396.
Winkler, J. D., Mong, S., and Crooke, S. T. 1988. Leukotriene D4-induced homologous desensitization of calcium mobilization in rat basophilic leukemia cells. J. Pharmacol. Exp. Ther. 244: 449–455.
Lombardini, J. B. 1998. Increased phosphorylation of specific rat cardiac and retinal proteins in taurine-depleted animals: Isolation and identification of the phosphoproteins. Adv. Exp. Med. Biol. 442:441–447.
Rabin, B., Nicolosi, R. J., and Hayes, K. C. 1976. Dietary influence on bile acid conjugation in the cat. J. Nutr. 106:1241–1246.
Hofmann, A. F. and Roda, A. 1984. Physicochemical properties of bile acids and their relationship to biological properties: An overview of the problem. J. Lipid Res. 25:1477–1489.
Huxtable, R. J. 2000. Expanding the circle 1975-1999: Sulfur biochemistry and insights on the biological functions of taurine. Adv. Exp. Med. Biol. 483:1–25.
Schaffer, S., Takahashi, K., and Azuma, J. 2000. Role of osmoregulation in the actions of taurine. Amino Acids 19:527–546.
Remy, A., Henry, S., and Tappaz, M. 1990. Specific antiserum and monoclonal antibodies against the taurine biosynthesis enzyme cysteine sulfinate decarboxylase: Identity of brain and liver enzyme. J. Neurochem. 54:870–879.
De Luca, A., Pierno, S., and Camerino, D. C. 1996. Effect of taurine depletion on excitation-contraction coupling and Cl− conductance of rat skeletal muscle. Eur. J. Pharmacol. 296:215–222.
Lambert, I. H. and Hoffmann, E. K. 1991. The role of phospholipase-A2 and 5-lipoxygenase in the activation of K and Cl channels and the taurine leak pathway in Ehrlich ascites tumor cells. Acta Physiol. Scand. 143:33A.
Finkel, T. 2001. Reactive oxygen species and signal transduction. IUBMB Life 52:3–6.
Hensley, K., Robinson, K. A., Gabbita, S. P., Salsman, S., and Floyd, R. A. 2000. Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 28:1456–1462.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lambert, I.H. Regulation of the Cellular Content of the Organic Osmolyte Taurine in Mammalian Cells. Neurochem Res 29, 27–63 (2004). https://doi.org/10.1023/B:NERE.0000010433.08577.96
Issue Date:
DOI: https://doi.org/10.1023/B:NERE.0000010433.08577.96