Abstract
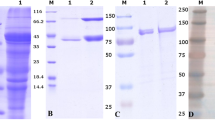
Mucus-bacterial interactions in the gastrointestinal tract and their impact on subsequent enteric infections are poorly delineated. In the present study, we have examined the binding ofSalmonella typhimurium to rat intestinal mucus and characterized a mucus protein (Mucus-Rs) which specifically binds to S. typhimurium. Both virulent (1402/84), and avirulent (SF 1835) S. typhimurium were observed to bind to crude mucus, however, the virulent strain showed 6 fold more binding as compared to avirulent strain. Fractionation of crude mucus on sepharose CL-6B resolved it into three major peaks. Maximal bacterial binding was observed with a high mol. wt. glycoprotein corresponding to neutral mucin. SDS-PAGE of purified protein (termed Mucus-Rs) under non reducing conditions showed it to be a homogenous glycoprotein (mol. wt. 250 kDa), while under reducing conditions, three bands corresponding to mol. wt. of 118,75 and 60 kDa were observed. Pretreatment of Mucus-Rs with pronase, trypsin and sodium metaperiodate markedly inhibited bacterial binding. GLC analysis of Mucus-Rs showed it to contain Mannose, Glucose, Galactose, Glucosamine, Galactosamine and Sialic acid as main sugars. Competitive binding in the presence of various sugars and lectins indicated the involvement of mannose in the mucus-bacterial interactions. The Mucus-Rs binding was highly specific for S. typhimurium; no significant binding was seen with E.coliand V. cholerae. Thus, we conclude that S. typhimurium specifically binds to a 250 kDa neutral mucin of intestinal tract. This binding appears to occur via specific adhesin-receptor interactions involving bacterial pili and mannose of neutral mucin.
Similar content being viewed by others
References
Labrec EH, Schneider H, Magnaric TS, Formal SB. Epithelial cell penetration as an essential step in the pathogenesis of bacillary dysentery. J Bacteriol 88: 1503-1508, 1964
Takeuchi A. Electron microscopic studies of experimental Salmonella infection: Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol 50: 109-136, 1967
Gianella RA, Formal SB, Dammin GJ, Collins H. Pathogenesis of Salmonellosis: Studies of fluid secretion mucosal invasion and morphologic reaction in the rabit ileum. J Clin Invest 52: 441-453, 1973
Forstner JF. Intestinal mucins in health and disease. Digestion 17: 234-263, 1978
Freter R. Mechanism of association of bacteria with mucosal surfaces. p. 36-55. In K. Elliot (ed.), Adhesion and microorganism pathogenecity. Ciba Foundation Symp. 80. Pitman Medical, Tunbridge Wells, UK, 1981
Mantle M, Basaraba L, Peacock SC, Gall DG. Binding of Yersinia enterocolitica to rabbit intestinal brush border membranes, mucus and mucin. Infect Immun 1989; 57: 3292-3299, 1989
McSweegan E, Walker RI. Identification and characterization of two campylobacter jejuni adhesins for cellular and mucous substrate. Infect Immun 53: 141-148, 1986
Sajjan SO, Reisman J, Doig P, Irvin RT, Forstner JF. Binding of nonmucoid pseudomonas aeruginosa to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Invest 89: 657-665, 1992
Sylvester FA, Philpott D, Benjamin G, Lastovica A, Forstner JF. Adherence to lipids and intestinal mucin by a recently recognized human pathogen, campylobacter upsaliensis. Infect Immun 64: 4060-4066, 1996
Venegas HF, Navas EL, Gaffney RA, Duncan JL, Anderson BE, Schaeffer AJ. Binding of type I, piliated E. coli to vaginal mucus. Infect Immun 63: 416-422, 1995
Wanke CA, Cronan S, Goss C, Chadee K, Guerrant RL. Characterization of binding of E. coli strains which are enteropathogens to small bowel mucin. Infect Immun 58: 794-800, 1990
Zenian A, Gillin FD. Interaction of Giardia lamblia with human intestinal mucus; Enhancement of trophozoite attachment to glass. J Protozool 32: 664-668, 1985
Nevola JJ, Stocker BAD, Laux DC, Cohen PS. Colonization of the mouse intestine by an avirulent Salmonella typhimurium strain and its LPS defective mutants. Infect Immun 50: 152-159, 1985
Nevola JJ, Laux DC, Cohen PS. In vivo colonization of the mouse large intestine and in vitro penetration of intestinal mucus by an avirulent smooth strain of Salmonella typhimurium and its lipopolysaccharide–deficient mutant. Infect Immun 55: 2884-2890, 1987
McCormick BA, Stocker BAD, Laux DC, Cohen PS. Roles of motility–chemotoxin and penetration through and growth in intestinal mucus in the ability of an avirulent strain of Salmonella typhimurium to colonize the large intestine of streptomycin treated mice. Infect Immun 56: 2209-2217, 1988
Rask Licht, Kasen AK, Paul S, Cohen, Lars KP, John U, Soren M. Role of lipopolysaccharide in colonization of the mouse intestine by Salmonella typhimurium studied by in situ hybridization. Infect Immun 64 (9): 3811-3817, 1996
Laux DC, McSweegan EF, Williams TJ, Wadolkowski EA, Cohen DS. Identification and characterization of mouse small intestinal mucosal receptors for E. coli. K-12 (K88ab). Infect Immun 52: 18-25, 1986
Lowry OH, Rosenbrough NJ, Farr AC, Randall GJ. Protein estimation with folin-phenol reagent. J Biol Chem 193: 265-275, 1951
Lindquist BL, Labenthal E, Lee PC, Stinson MW, Merrick JM. Adherence of Salmonella typhimurium to small intestinal enterocytes of the rat. Infect Immun 53: 3044-3050, 1987
Laux DC, McSweegan EF, Cohen PS. Adhesion of enterotoxigenic E. coli to immobilized intestinal mucosal preparations: A model for adhesion to mucosal surface components. J Microbiol Methods 2: 27-39, 1984
Paeregaard A, Espersen F, Jenson OM, Skurnik M. Interactions between Yersinia enterocolitica and rabbit ileal mucus growth, adhesion, penetration and subsequent changes in surface hydrophobicity and ability to adhere to ileal brush border membrane vesicles. Infect Immun 59: 253-260, 1991
Gupta Y, Chand P, Singh A, Jain NC. Dot immunobinding assay in comparison with enzyme linked immunosorbent assay for the detection of blue tongue virus in sheep. Vet Microbiol 22: 365-371, 1990
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277: 680-685, 1970
Dubray G, Bezard G. A highly sensitive periodic acid–silver stain for 1,2–Diol groups of glycoproteins and polysaccharides in polyacrylamide Gels. Anal Biochem 119: 325-329, 1982
Towbin H. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets. Proc Natl Acad Sci USA 76: 4350-4354, 1979
Lasky RA, Mill DA. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem 56: 335-341, 1975
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith. Colorimetric method for determination of sugars and related substances. Analyst Chemistry 28: 350-355, 1956
Svennerholm L. Quantitative estimation of sialic acid II. A colorimetric resorcinol–hydrochloric acid method. Biochem Biophys Acta 24: 604-611, 1957
Cohen PS, Arruda JC, Williams TJ, Laux DC. Adhesion of a human fecal E. coli strain to mouse colonic mucus. Infect Immun 48: 139-145, 1985
Falk P, Roth KA, Boren T, Westblom TU, Gordon JI, Normak S. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell linkage specific tropism in the human gastric epithelium. Proc Natl Acad Sci USA 90: 2035-2039, 1993
Folch J, Lees M, Sloane SGH. A simple method for isolation and purification of total lipids from animal tissue. J Biol Chem 226: 497-509, 1957
Karl-Anders Karlsson. Animal glycosphingolipids as membrane attachment sites for bacteria. Ann Rev Biochem 58: 309-350, 1989
Karlsson KA, Stromberg N. Overlay and solid phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol 138: 220-232, 1987
Mantle M, Husar SD. Adhesion of Yersinia enterocolitica to purified rabbit and human intestinal mucin. Infect Immun 61: 2340-2346, 1993
Blomberg L, Krivan HC, Cohen PS, Conway PL. Piglet ileal mucus contains protein and glycolipid (Galactosyl ceramide) receptors specific for E. coli K88 fimbriae. Infect Immun 61: 2526-2531, 1993
Khan AS, Johnson NC, Goldfine H, Schifferli BM. Porcine 987P Glycolipid receptors on intestinal brush borders and their cognate bacterial ligands. Infect Immun 64: 3688-3693, 1996
Fahmin REF, Specian RD, Forstner GG, Forstner JF. Characterization and localization of the putative 'link' component in rat small intestinal mucin. Biochem J 243: 631-640, 1987
Sajjan SU, Forstner JF. Role of putative 'link' glycopeptide of intestinal mucin in binding of piliated E. coli serotype 0157: H7 strain CL-49. Infect Immun 58: 868-873, 1990
Brandley BK, Swiedler SJ, Robbins PW. Carbohydrate ligand of the LEC cell adhesion molecules. Cell 63: 861-863, 1990
Polley MJ, Philips LM, Wayner E, Nudelman E, Singhal AK, Hakomori S, Paulson JC. CD62 and endothelial cell leukocyte adhesin molecule 1 (ELAM-1) recognize the same carbohydrate ligand sialyl–Lewis X. Proc Natl Acad Sci USA 88: 6224-6228, 1991
Ghosh S, Mittal A, Ganguly NK. Purification and characterization of destinct type of mannose sensitive fimbriae for S. typhimurium. Mol Cell Biochem 115: 229-234, 1994
Clegg S, Gerlach GF. Enterobacterial fimbrial. J Bacteriol 169: 934-948, 1987
Knibbs RN, Goldstein IJ, Ratcliffe RM, Shibuya N. Characterization of carbohydrate binding specificity of the leukoagglutinating lectin from maackia emurensis. J Biol Chem 266: 83-88, 1991
Giannasca KT, Giannasca PJ, Neutra MR. Ahderence of Salmonella typhimurium to CaCO2-2 cells; Identification of a Glycoconjugate receptor. Infect Immun 64: 135-145, 1996
Sujata G, Mittal A, Vohra H, Ganguly NK. Interaction of rat intestinal brush border membrane glycoprotein with type-1 fimbriated of Salmonella typhimurium. Molecular and Cellular Biochemistry 158: 125-131, 1996
Ensgraber M, Loos M. A 66 kilodalton heat shock protein of Salmonella typhimurium is responsible for binding of the bacterium to mucus. Infect Immun 60: 3072-3078, 1992
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vimal, D., Khullar, M., Gupta, S. et al. Intestinal mucins: the binding sites forsalmonella typhimurium. Mol Cell Biochem 204, 107–117 (2000). https://doi.org/10.1023/A:1007015312036
Issue Date:
DOI: https://doi.org/10.1023/A:1007015312036