Abstract
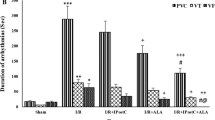
Diabetic hearts are suggested to exhibit either increased or lower sensitivity to ischemia. Detrimental effects of prolonged ischemia can be attenuated by preconditioning, however, relatively little is known about its effects in the diseased myocardium. This study was designed to test the susceptibility to ischemia-induced arrhythmias and the effect of preconditioning in the diabetic heart. Rats were made diabetic with streptozotocin (45 mg/kg, i.v.). After 1 week, isolated Langendorff-perfused hearts were subjected to 30 min occlusion of LAD coronary artery without or with preceding preconditioning induced by one cycle of 5 min ischemia and 10 min reperfusion. Glycogen and lactate contents were estimated in the preconditioned and non-preconditioned hearts before and after ischemia. Diabetic hearts were more resistant to ischemia-induced arrhythmias: incidence of ventricular tachycardia (VT) decreased to 42% and only transient ventricular fibrillation (VF) occurred in 17% of the hearts as compared to the non-diabetic controls (VT 100% and VF 70% including sustained VF 36%; p < 0.05). Preconditioning effectively suppressed the incidence and severity of arrhythmias (VT 33%, VF 0%) in the normal hearts. However, this intervention did not confer any additional protection in the diabetic hearts. Despite higher glycogen content in the diabetic myocardium and greater glycogenolysis during ischemia, production of lactate in these hearts was significantly lower than in the controls. Preconditioning caused a substantial decrease in the accumulation of lactate in the normal hearts, whereby in the diabetic hearts, this intervention did not cause any further reduction in the level of lactate. In conclusion, diabetic rat hearts exhibit lower susceptibility to ischemic injury and show no additional response to preconditioning. Reduced production of glycolytic metabolites during ischemia can account for the enhanced resistance of diabetic hearts to ischemia as well as for the lack of further protection by preconditioning.
Similar content being viewed by others
References
Dubrey SW, Reaveley DR, Seed M, Lane DA, Ireland H, O'Donnell M, O'Connor B, Noble MI, David R, Leslie G: Risk factors for cardiovascular disease in IDDM. A study of identical twins. Diabetes 43: 831–835, 1994
Kannel WB, McGee DL: Diabetes and cardiovascular risk factors. The Framingham Study. Circulation 59: 8–13, 1979
Dhalla NS, Liu X, Panagia V, Takeda A: Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res 40: 239–247, 1998
Ganguly PK, Pierce GN, Dhalla KS, Dhalla NS: Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol 244: E528–E535, 1983
Khandoudi A, Bernard M, Cozzone P, Feuvray D: Intracellular pH and role of Na+/H+ exchange during ischemia and reperfusion of normal and diabetic rat hearts. Cardiovasc Res 24: 873–878, 1990
Pierce GN, Ramjiawan B, Dhalla NS, Ferrari R: Na+/H+ exchange in cardiac sarcolemmal vesicles isolated from diabetic rats. Am J Physiol 258: H255–E261, 1990
Dhalla NS, Pierce GN, Panagia V, Singal PK, Beamish RE: Calcium movements in relation to heart function. Basic Res Cardiol 77: 117–139, 1982
Opie LH: Myocardial ischemia, reperfusion and cytoprotection. Rev Port Cardiol 15: 703–708, 1996
Sulova Z, Vyskocil F, Stankovicova T, Breier A: Ca2+-induced inhibition of sodium pump: Effects on energetic metabolism of mouse diaphragm tissue. Gen Physiol Biophys 17: 271–283, 1998
Forrat R, Sebbag L, Wiensperger A, Guidollet J, Delaye J, De Lorgeril M: Acute myocardial infarction in diabetic dogs with experimental diabetes. Cardiovasc Res 27: 1908–1912, 1993
Hadour G, Ferrera R, Sebbag L, Forrat R, Delaye J, De Lorgeril M: Improved myocardial tolerance to ischemia in the diabetic rabbit. J Mol Cell Cardiol 30: 1869–1875, 1998
Liu Y, Thornton JD, Cohen MV, Downey JM, Schaffer SW: Streptozotocin-induced non-insulin dependent diabetes protects the heart from infarction. Circulation 88: 1273–1278, 1993
Hekimian G, Khandoudi N, Feuvray D, Beigelman PM: Abnormal cardiac rhythm in diabetic rats. Life Sci 37: 547–551, 1985
Kusama Y, Hearse DJ, Avkiran M: Diabetes and susceptibility to reperfusion-induced ventricular arrhythmias. J Mol Cell Cardiol 24: 411–421, 1992
Tani M, Neely JR: Hearts from diabetic rats are more resistant to in vitro ischemia: Possible role of altered Ca2+ metabolism. Circ Res 62: 931–940, 1988
Tosaki A, Engelman DT, Engelman RM, Das DK: The evolution of diabetic response to ischemia/reperfusion and preconditioning in isolated working rat hearts. Cardiovasc Res 31: 526–536, 1996
Ravingerova T, Styk J, Pancza D, Tribulova A, Sebokova J, Volkovova K, Ziegelhoffer A, Slezak J: Diabetic cardiomyopathy in rats: alleviation of myocardial dysfunction caused by Ca2+ overload. Diabetes Res Clin Practice 31: S105–S112, 1996
Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986
Liu GS, Downey JM: Preconditioning against infarction in rat heart does not involve pertussis toxin sensitive G protein. Cardiovasc Res 27: 608–611, 1993
Cave AC: Preconditioning induced protection against postischemic contractile dysfunction: Characteristics and mechanisms. J Mol Cell Cardiol 27: 969–979, 1995
Vegh A, Komori S, Szekeres L, Parratt JR: Antiarrhythmic effects of preconditioning in anaesthetised dogs and rats. Cardiovasc Res 26: 487–495, 1992
Bouchard JF, Lamontagne D: Protection afforded by preconditioning in the diabetic heart against ischemic injury. Cardiovasc Res 37: 82–90, 1998
Curtis MJ: Characterisation, utilisation and clinical relevance of isolated perfused heart models of ischemia-induced ventricular fibrillation. Cardiovasc Res 39: 194–215, 1998
Ravingerova T, Tribulova N, Slezak J, Curtis MJ: Brief, intermediate and prolonged ischemia in the crystalloid perfused rat heart: Relationship between susceptibility to arrhythmias and degree of ultrastructural injury. J Mol Cell Cardiol 27: 1937–1951, 1995
Walker MJA, Curtis MJ, Hearse DJ, Campbell RWF, Janse MJ, Yellon DM, Cobbe SM, Coker SJ, Harness JB, Harron DWG, Higgins AJ, Julian DJ, Lab MJ, Manning AS, Northover BJ, Parratt JR, Riemersma RA, Riva E, Russel DC, Sheridan DJ, Winslow E, Woodward B: The Lambeth conventions: Guidelines for the study of arrhythmias in ischemia, infarction, and reperfusion. Cardiovasc Res 22: 447–455, 1988
Ravingerova T, Pyne NJ, Parratt JR: Ischaemic preconditioning in the rat heart: The role of G-proteins and adrenergic stimulation. Mol Cell Biochem 147: 123–128, 1995
Fedelesova M, Ziegelhö ffer A, Krause E-G, Wollenberger A: Effect of exogenous adenosine triphosphate on the metabolic state of the excised hypothermic dog heart. Circulation Res 24: 617–627, 1969
Ravingerova T, Stetka R, Volkovova K, Pancza D, Ziegelhoffer A, Styk J: Responses to ischaemia and to preconditioning are modified in the diabetic rat hearts. J Mol Cell Cardiol 31: A98, 1999
Curtis MJ, Hearse DJ: Ischemia-induced and reperfusion-induced arrhythmias differ in their sensitivity to potassium: Implications for the mechanisms of initiation and maintenance of ventricular fibrillation. J Mol Cell Cardiol 21: 21–40, 1989
Shimoni Y, Severson D, Giles W: Thyroid status and diabetes modulate regional differences in potassium currents in rat ventricle. J Physiol (Lond) 488: 673–688, 1995
Smith JM, Wahler GM: ATP-sensitive potassium channels are altered in ventricular myocytes from diabetic rats. Mol Cell Biochem 158: 43–51, 1996
Noma A: ATP-regulated K+ channels in cardiac muscle. Nature 305: 147–148, 1983
Spinelli W, Sorota S, Siegal M, Hoffman BF: Antiarrhythmic actions of the ATP-regulated K+ current activated by pinacidil. Circ Res 68: 1127–1137, 1991
Rodrigues B, Cam MC, McNeill JH: Myocardial substrate metabolism: Implications for diabetic cardiomyopathy. J Mol Cell Cardiol 27: 169–179, 1995
Goto M, Tsuchida A, Liu Y, Cohen MV, Downey JM: Transient inhibition of glucose uptake mimics ischemic preconditioning by salvaging ischemic myocardium in the rabbit heart. J Mol Cell Cardiol 27: 1883–1894, 1995
Feuvray D, Lopaschuk GD: Controversies on the sensitivity of the diabetic heart to ischemic injury: The sensitivity of the diabetic heart to ischemic injury is decreased. Cardiovasc Res 34: 113–120, 1997
Gamble J, Lopaschuk GD: Glycolysis and glucose oxidation during reperfusion of ischemic hearts from diabetic rats. Biochim Biophys Acta Mol Basis Dis 1225: 191–199, 1994
Meldrum DR, Cleveland JC Jr, Sheridan BC, Rowland RT, Banerjee A, Harken AH: Cardiac preconditioning with calcium: Clinically accessible myocardial protection. J Thorac Cardiovasc Surg 112: 778–786, 1996
Malhotra A, Reich D, Reich D, Nakouzi A, Sanghi V, Geenen DL, Buttrick PM: Experimental diabetes is associated with functional activation of protein kinase Ce and phosphorylation of troponin I in the heart, which are prevented by angiotensin II receptor blockade. Circ Res 81: 1027–1033, 1997
Mitchell MB, Meng X, Ao L, Brown JM, Harken AH, Banerjee A: Preconditioning of isolated rat heart is mediated by protein kinase C. Circ Res 76: 73–81, 1995
Gross GJ, Auchampach JA: Blockade of ATP-sensitive potassium channel prevents myocardial preconditioning in dogs. Circ Res 70: 223–233, 1992
Botsford MW, Lukas A: Ischemic preconditioning and arrhythmogenesis in the rabbit heart: Effects on epicardium vs. endocardium. J Mol Cell Cardiol 30: 1723–1735, 1998
Tan HL, Mazon P, Verberne HJ, Sleeswijk ME, Coronel R, Opthof T, Janse M: Ischemic preconditioning delays ischemia induced cellular electrical uncoupling in rabbit myocardium by activation of ATP sensitive potassium channels. Cardiovasc Res 27: 644–651, 1993.
Wolfe CL, Sievers RE, Visseren FLJ, Donnelly TJ: Loss of myocardial protection after preconditioning correlates with the time course of glycogen recovery within the preconditioned segment. Circulation 87: 881–892, 1993
Tatsumi T, Matoba S, Kobara M, Keira N, Kawahara A, Tsuruyama K, Tanaka T, Katamura M, Nakagawa C, Ohta B, Yamahara Y, Asayama J, Nakagawa M: Energy metabolism after ischemic preconditioning in streptozotocin-induced diabetic rat hearts. J Am Coll Cardiol 31: 707–715, 1998
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ravingerova, T., Stetka, R., Volkovova, K. et al. Acute diabetes modulates response to ischemia in isolated rat heart. Mol Cell Biochem 210, 143–151 (2000). https://doi.org/10.1023/A:1007129708262
Issue Date:
DOI: https://doi.org/10.1023/A:1007129708262