Abstract
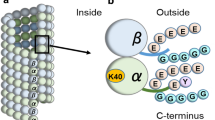
Tubulin normally undergoes a cycle of detyrosination/tyrosination on the carboxy terminus of its α-subunit and this results in subpopulations of tyrosinated tubulin and detyrosinated tubulin. Brain tubulin preparations also contain a third major tubulin subpopulation which is non-tyrosinatable. This review describes the purification and the structural characterization of non-tyrosinatable tubulin. This tubulin variant lacks a carboxyterminal glutamyl-tyrosine group on its α-subunit (Δ2-tubulin). Δ2-tubulin is generated from detyrosinated tubulin through an irreversible reaction. Δ2-tubulin accumulates in neurons and in stable microtubule assemblies. It also accumulates in some tumor cells due to the frequent loss of tubulin tyrosine ligase in such cells. Δ2-tubulin may be a useful marker of malignancy in human tumors.
Similar content being viewed by others
REFERENCES
LeDizet, M., and Piperno, G. 1987. Identification of an acetylation site of Chlamydomonas alpha-tubulin. Proc. Nat. Acad. Sci. USA 84:5720-5724.
Eddé, B., Rossier, J., Le Caer, J. P., Desbruyè res, E., Gros, F., and Denoulet, P. 1990. Posttranslational glutamylation of alphatubulin. Science 247:83-85.
Alexander, J. E., Hunt, D. F., Lee, M. K., Shabanowitz, J., Michel, H., Berlin, S. C., MacDonald, T. L., Sundberg, R. J., Rebhun, L. I., and Frankfurter, A. 1991. Characterization of posttranslational modifications in neuron-specific class III β-tubulin by mass spectrometry. Proc. Nat. Acad. Sci. USA 88:4685-4689.
Arce, C. A., Rodriguez, J. A., Barra, H. S., and Caputto, R. 1975. Incorporation of L-tyrosine, L-phenylalanine and L-3,4-dihydroxyphenylalanine as single units into rat brain tubulin. Eur. J. Biochem. 59:145-149.
Argarañ a, C. E., Arce, C. A., Barra, H. S., and Caputto R. 1977. In vivo incorporation of [14C] tyrosine into the C-terminal position of the α-subunit of tubulin. Arch. Biochem. Biophys. 180:264-268.
Raybin, D., and Flavin, M. 1977a. Enzyme which specifically adds tyrosine to the alpha chain of tubulin. Biochemistry 16:2189-2194.
Wehland, J., Schroeder, H. C., and Weber, K. 1986. Isolation and purification of tubulin tyrosine ligase. Meth. Enzymol. 134:170-179.
Barra, H. S., Arce, C. A., and Argarañ a, C. E. 1988. Posttranslational tyrosination/detyrosination of tubulin. Mol. Neurobiol. 2:133-153.
MacRae, T. H. 1997. Tubulin post-translational modificationsenzymes and their mechanisms of action. Eur. J. Biochem. 244:265-278.
Barra, H. S., Uñ ates, L. E., Sayavedra, M., and Caputto, R. 1972. Capacities for binding amino acids by tRNAs from rat brain and their changes during development. J. Neurochem. 19:2289-2297.
Barra, H. S., Rodriguez, J. A., Arce, C. A., and Caputto, R. 1973a. A soluble preparation from rat brain that incorporates into its own proteins [14C] arginine by ribonuclease-sensitive system and [14C] tyrosine by a ribonuclease-insensitive system. J. Neurochem. 20:97-108.
Barra, H. S., Arce, C. A., Rodriguez, J. A., and Caputto, R. 1973b. Incorporation of phenylalanine as single unit into rat brain protein: Reciprocal inhibition by phenylalanine and tyrosine of their respective incorporations. J. Neurochem. 21:1241-1251.
Barra, H. S., Arce, C. A., Rodriguez, J. A., and Caputto, R. 1974. Some common properties of the protein that incorporates tyrosine as a single unit and the microtubule proteins. Biochem., Biophys. Res. Commun. 60:1384-1390.
Hallak, M. E., Rodriguez, J. A., Barra, H. S., and Caputto, R. 1977. Release of tyrosine from tyrosinated tubulin. Some common factors that affect this process and the assembly of tubulin. FEBS Lett. 73:147-150.
Argarañ a, C. E., Barra, H. S., and Caputto, R. 1978. Release of [14C]tyrosine from tubulinyl[14C]tyrosine by brain extract. Separation of a carboxypeptidase from tubulin:tyrosine ligase. Mol. Cell. Biochem. 19:17-21.
Argarañ a, C. E., Barra, H. S., and Caputto, R. 1980. Tubulinyltyrosine carboxypeptidase from chicken brain: properties and partial purification. J. Neurochem. 34:114-118.
Little, M., and Seehaus, T. 1988. Comparative analysis of tubulin sequences. Comp. Biochem. Physiol. 90B:655-670.
Preston, S. F., Deanin, G. G., Hanson, R. D., and Gordon, M. W. 1979. The phylogenic distribution of tubulin:tyrosine ligase. J. Mol. Evol. 13:233-244.
Kobayashi, T., and Flavin, M. 1981. Tubulin tyrosylation in invertebrates. Comp. Biochem. Physiol. 69B:387-392.
Thompson, W. C. 1982. The cyclic tyrosination/detyrosination of alpha tubulin. Meth. Cell Biol. 24:235-255.
Gabius, H. J., Graupner, G., and Cramer, F. 1983. Activity patterns of aminoacyl-tRNA synthetases, tRNA methylases, arginyltransferases and tubulin:tyrosine ligase during development and ageing of Caenorhabditis elegans. Eur. J. Biochem. 131:231-234.
Steiger, J., Wyler, T., and Seebeck, T. 1984. Partial purification and characterization of microtubular protein from Trypanosoma brucei. J. Biol. Chem. 259:4596, 4602.
Schröder, H. C., Wehland, J., and Weber, K. 1985. Purification of brain tubulin:tyrosine ligase by biochemical and immunological methods. J. Cell Biol. 100:276-281.
Ersfeld, K., Wehland, J., Plessman, U., Dodemont, H., Gerke, V., and Weber, K. 1993. Characterization of the tubulin-tyrosine ligase. J. Cell Biol. 120:725-732.
Arce, C. A., Hallak, M. E., Rodriguez, J. A., Barra, H. S., and Caputto, R. 1978. Capability of tubulin and microtubules to incorporate and to release tyrosine and phenylalanine and the effect of the incorporation of these amino acids on tubulin assembly. J. Neurochem. 31:205-210.
Rodriguez, J. A., and Borisy, G. G. 1979. Tyrosination state of free tubulin subunits and tubulin disassembled from microtubules of rat brain tissue. Biochem. Biophys. Res. Commun. 83:579-586.
Beltramo, D. M., Arce, C. A., and Barra, H. S. 1987. Tubulin but not microtubules is the substrate of tubulin:tyrosine ligase in mature avian erythrocytes. J. Biol. Chem. 262:15673-15677.
Bré, M. H., Kreis, T. E., and Karsenti, E. 1987. Control of microtubule nucleation and stability in Madin-Darby canine kidney cells: the occurence of noncentrosomal, stable detyrosinated microtubules. J. Cell Biol. 105:1283-1296.
Gundersen, G. G., Khawaja, S., and Bulinski, J. C. 1987. Postpolymerisation detyrosination of alpha-tubulin: a mechanism for subcellular differentiation of microtubules. J. Cell Biol. 105:251-264.
Kumar, N., and Flavin, M. 1981. Preferential action of brain detyrosinolating carboxypeptidase on polymerized tubulin. J. Biol. Chem. 256:7678-7686.
Arce, C. A., and Barra, H. S. 1983. Association of tubulinyl-tyrosine carboxypeptidase with microtubules. FEBS Lett. 157: 75-78.
Arce, C. A., and Barra, H. S. 1985. Release of C-terminal tyrosine from tubulin and microtubules at steady state. Biochem. J. 226:311-317.
Sironi, J. J., Barra, H. S., and Arce, C. A. 1997. The association of tubulin carboxypeptidase activity with microtubules in brain extracts is modulated by phosphorylation/dephosphorylation processes. Mol. Cell. Biochem. 170:9-16.
Wehland, J., Willingham, M. C., and Sandoval, I. V. 1983. A rat monoclonal antibody reacting specifically with the tyrosylated form of alpha-tubulin. I. Biochemical characterization, effects on microtubule polymerisation in vitro, and microtubule polymerisation and organization in vivo. J. Cell Biol. 97:1467-1475.
Gundersen, G. G., Kalnoski, M. H., and Bulinski, J. C. 1984. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell 38:779-789.
Gundersen, G. G., and Bulinski, J. C. 1986. Microtubule arrays in differentiated cells contain elevated levels of post-translationally modified form of tubulin. Eur. J. Cell Biol. 42:288-294.
Kreis, T. E. 1987. Microtubules containing detyrosinated tubulin are less dynamic. EMBO J. 6:2597-2606.
Schulze, E., Asai, D., Bulinski, J. C., and Kirschner, M. 1987. Posttranslational modification and microtubule stability. J. Cell Biol. 105:2167-2177.
Wehland, J., and Weber, K. 1987b. Turnover of the carboxyterminal tyrosine of alpha-tubulin and means of reaching elevated levels of detyrosination in living cells. J. Cell Sci. 88:185-203.
Bosc, C., Cronk, J. D., Pirollet, F., Watterson, D. M., Haiech, J., Job, D., and Margolis, R. L. 1996. Cloning, expression, and properties of the microtubule-stabilizing protein STOP. Proc. Nat. Acad. Sci. USA. 93:2125-2130.
Deanin, G. G., Preston, S. F., Hanson, R. K., and Gordon, M. W. 1980. On the mechanism of turnover of the carboxy-terminal tyrosine of the alpha chain of tubulin. Eur. J. Biochem. 109:207-216.
Modesti, N. M., Argarañ a, C. E., Barra, H. S., and Caputto, R. 1984. Inhibition of brain tubulinyl-tyrosine carboxypeptidase by endogeneous proteins. J. Neurosci. Res. 12:583-593.
Webster, D. R., Modesti, N. M., and Bulinski, J. C. 1992. Regulation of cytoplasmic tubulin carboxypeptidase activity during neural and muscle differentiation: characterization using a microtubule based assay. Biochemistry 31:5849-5856.
Webster, D. R., and Oxford, M. G. 1996. Regulation of cytoplasmic tubulin carboxypeptidase activity in vitro by cations and sulfhydryl-modifying compounds. J. Cell Biochem. 60:424-436.
Paturle, L., Wehland, J., Margolis, R. L., and Job, D. 1989. Complete separation of tyrosinated, detyrosinated, and nontyrosinatable brain tubulin subpopulations using affinity chromatography. Biochemistry 28:2698-2704.
Khawaja, S., Gundersen, G. G., and Bulinski, J. C. 1988. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J. Cell Biol. 106:141-149.
Webster, D. R., Wehland, J., Weber, K., and Borisy, G. G. 1990. Detyrosination of alpha tubulin does not stabilize microtubules in vivo. J. Cell Biol. 111:113-122.
Raybin, D., and Flavin, M. 1977b. Modification of tubulin by tyrosylation in cells and extracts and its effect on assembly in vitro. J. Cell Biol. 73:492-504.
Rodriguez, J. A., and Borisy, G. G. 1978. Modification of the C-terminus of brain tubulin during development. Biochem. Biophys. Res. Commun. 83:579-586.
Barra, H. S., Arce, C. A., and Caputto, R. 1980. Total tubulin and its aminoacylated and non-aminoacylated forms during the development of rat brain. Eur. J. Biochem. 109: 439-446.
Flavin, M., Kobayashi, T., and Martensen, T. M. 1982. Tubulintyrosine ligase from brain. Methods Cell Biol. 24:257-263.
Villasante, A., Wag, D., Dobner, P., Dolph, P., Lewis, S. A., and Cowan, N. J. 1986. Six mouse α-tubulin mRNAs encode five distinct tubulin isotypes: testis-specific expression of two sister genes. Mol. Cell. Biol. 6:2409-2419.
Gu, W. Lewis, S. A., and Cowan, N. J. 1988. Generation of antisera that discriminate among mammalian α-tubulins: introduction of specialized isotypes into cultured cells results in their coassembly without disruption of normal microtubule function. J. Cell Biol. 106:2011-2022.
Wehland, J., and Weber, K. 1987a. Tubulin-tyrosine ligase has a binding site on beta-tubulin: a two-domain structure of the enzyme. J. Cell Biol. 104:1059-1067.
Wehland, J., Schroeder, H. C., and Weber, K. 1984. Amino acid requirements in the epitope recognized by the α-tubulinspecific rat monoclonal antibody YL 1/2. EMBO J. 3:1295-1300.
Patude-Lafanechè re, L., Eddé, B., Denoulet, P., Van Dorsselaer, A., Mazarguil, H., Le Caer, J. P., Wehland, J., and Job, D. 1991. Characterization of a major brain tubulin variant which cannot be tyrosinated. Biochemistry 30:10523-10528.
Rüdiger, M., Wehland, J., and Weber, K. 1994. The carboxyterminal peptide of detyrosinated αtubulin provides a minimal system to study the substrate specificity of tubulin-tyrosine ligase. Eur. J. Biochem. 220:309-320.
Paturle-Lafanechè re, L., Manier, M., Trigault, N., Pirollet, F., Mazarguil, H., and Job, D. 1994. Accumulation of delta 2-tubulin, a major tubulin variant that cannot be tyrosinated, in neuronal tissues and in stable microtubule assemblies. J. Cell Sci. 107:1529-1543.
Alonso, A., Del, C., Arce, C. A., and Barra, H. S. 1993. Tyrosinatable and non-tyrosinatable tubulin subpopulations in rat muscle in comparison with those in brain. Biochim. Biophys. Acta 1163:26-30.
Lafanechè re, L., Courtay-Cahen, C., Kawakami, T., Jacrot, M., Rüdiger, M., Wehland, J., Job, D., and Margolis, R. L. 1998. Suppression of tubulin tyrosine ligase during tumor growth. J. Cell Sci. 111:171-181.
Guillaud, L., Bosc, C., Fourest-Lieuvin, A., Denarier, E., Pirollet, F., Lafanechè re, L., and Job, D. 1998. STOP proteins are responsible for the high degree of microtubule stabilization observed in neuronal cells. J. Cell Biol. 142:167-179.
Mary, J., Redeker, V., Le Caer, J. P., Rossier, J., and Schmitter, J. M. 1996. Posttranslational modifications in the C-terminal tail of axonemal tubulin from sea urchin sperm. J. Biol. Chem. 271:9928-9933.
Multigner, L., Pignot-Paintrand, I., Saoudi, Y., Job, D., Plessman, U., Rüdiger, M., and Weber, K. 1996. The A and B tubules of the outer doublets of sea urchin sperm axonemes are composed of different tubulin variants. Biochemistry 33:10862-10871.
Sato, H., Nagai, T., Kuppuswamy, D., Narishige, T., Koide, M., Menick, D. R., and Cooper IV, G. 1997. Microtubule stabilization in pressure overload cardiac hypertrophy. J. Cell Biol. 139:963-973.
Smertenko, A., Blume, Y., Viklicy, V, Opatrny, Z., and Draber, P. 1997. Post-translational modifications and multiple tubulin isoforms in Nicotinia tabacum L. cells. Planta 201:349-358.
Manier, M., Christina, N., Chatellard-Causse, C., Mouchet, P., Herman, J. P., and Feuerstein, C. 1997. Striatal target-induced axonal branching of dopaminergic mesenphalic neurons in culture via diffusible factors. J. Neurosci. Res. 48:358-371.
Maxwell, S. A., Ames, S. K., Sawai, E. T., Decker, G. L., Cook, R. G., and Butel, J. S. 1991. Simian virus 40 large T antigen and p53 are microtubule-associated proteins in transformed cells. Cell growth Differ. 2:115-127.
Reszka, A. A., Seger, R., Diltz, C. D., Krebs, E. G., and Fisher, E. H. 1995. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc. Nat. Acad Sci. USA 92:8881-8885.
Bershadsky, A., Chausovsky, A., Becker, E., Lyubimova, A., and Geiger, B. 1996. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr. Biol. 6:1279-1289.
Murphy, M., Hinman, A., and Levine, A. J. 1996. Wild-type p53 negatively regulates the expression of a microtubule-associated protein. Genes Dev. 10:2971-2980.
Thomas, R. C., Edwards, M. J., and Marks, R. 1996. Translocation of the retinoblastoma gene product during mitosis. Exp. Cell Res. 223:227-232.
Trielli, M. O., Andreassen, P. R., Lacroix, F. B., and Margolis, R. L. 1996. Differential taxol arrest of transformed and nontransformed cells in the G1 phase of the cell cycle, and specific-related mortality of transformed cells. J. Cell Biol. 135:689-700.
Li, F., Ambrosini, G., Chu, E. Y., Plescia, J., Tognin, S., Marchisio, P. C., and Altieri, D. C. 1998. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 396:580-584.
Lingle, W. L., Lutz, W. H., Ingle, J. N., Nita, J. M., and Salisbury, J. L. 1998. Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc. Nat. Acad Sci. USA 95:2950-2955.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lafanechère, L., Job, D. The Third Tubulin Pool. Neurochem Res 25, 11–18 (2000). https://doi.org/10.1023/A:1007575012904
Issue Date:
DOI: https://doi.org/10.1023/A:1007575012904