Abstract
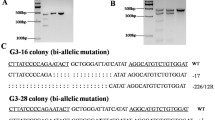
Miniature pig is an attractive animal for a wide range of research fields, such as medicine and pharmacology, because of its small size, the possibility of breeding it under minimum environmental controls and the physiology that is potentially similar to that of human. Although transgenic technology is useful for the analysis of gene function and for the development of model animals for various diseases, there have not yet been any reports on producing transgenic miniature pig. This study is the first successful report concerning the production of transgenic miniature pig by pronuclear microinjection. The huntingtin gene cloned from miniature pig, which is a homologue of candidate gene for Huntington's disease, connected with rat neuron-specific enolase promoter region, was injected into a pronucleus of fertilized eggs with micromanipulator. The eggs were transferred into the oviduct of recipient miniature pigs, whose estrus cycles were previously synchronized with a progesterone analogue. A total of 402 injected eggs from 171 donors were transferred to 23 synchronized recipients. Sixteen of them maintained pregnancy and delivered 65 young, and one resulted in abortion. Five of the 68 offspring (three of which were aborted) were determined to have transgene by PCR and Southern analysis. The overall rate of transgenic production was 1.24% (transgenic/injected eggs). This study provides the first success and useful information regarding production of transgenic miniature pig for biomedical research.
Similar content being viewed by others
References
Baker RD and Coggins EG (1968) Control of ovulation rate and fertilization in prepuberal gilts. J Anim Sci 27: 1607–1610.
Bollen P and Ellegaard L (1997) The Göttingen minipig in pharmacology and toxicology. Pharmacol Toxicol 80: 3–4.
Brem G, Springmann K, Meier E, Kräuβlich H, Brenig B, Müller M and Winnacker E-L (1990) Factors in the success of transgenic pig programs. In: Church RB (ed.), Transgenic Models in Medicine and Agriculture. Vol. 116 (pp. 61–72) Wiley-Liss, New York.
Britt JH, Day BN, Webel SK and Brauer MA (1989) Induction of fertile estrus in prepuberal gilts by treatment with a combination of pregnant mare's serum gonadotropin and human chorionic gonadotropin. J Anim Sci 67: 1148–1153.
Bustad LK and McClellan RO (1966) Swine in Biomedical Research. Battlle memorial institute, Seattle.
Cameron ER, Harvey and Onions DE (1994) Transgenic science. Br Vet J 150: 9–24.
Cozzi E and White DJG (1995) The generation of transgenic pigs as potential organ donors for humans. Nature Medicine 1(9): 964–966.
Cunningham PJ (1979) Selection for ovulation rate in swine: correlated response in litter size and weight. J Anim Sci 48: 509–516.
Douglas WR (1972) Of pigs and men and research: a review of applications and analogies of the Pig, sus scrofa, in human medical research. Space Life Sci 3: 226–234.
French AJ, Zviedrans P, Ashman RJ, Heap PA and Seamark RF (1991) Comparison of prepubertal and postpubertal young sows as a source of one-cell embryos for microinjection. Theriogenology vn35: 202.
Glodek P (1986) Breeding program and population standards of the Göttingen miniature swine. In: Tumbleson ME (ed.) Swine in Biomedical Research. Vol. I(pp. 23–28) Plenum Press, New York.
Hajdu MA, Knight JW, Canseco RS, Krisher RL, Velander WH, Pearson RE and Gwazdauskas FC (1994) Effect of culture conditions, donor age, and injection site on in vitro development of DNA microinjected porcine zygotes. J AnimSci 72: 1299–1305.
Hammer RE, Pursel VG, Rexroad Jr CE, Wall RJ, Bolt DJ, Ebert JM, Palmiter RD and Brinster RL (1985) Production of transgenic rabbits, sheep and pigs by microinjection. Nature 315: 680–683.
Hammer RE, Pursel VG, Rexroad Jr CE, Wall RJ, Bolt DJ, Palmiter RD and Brinster RL (1986) Genetic engineering of mammalian embryos. J Anim Sci 63: 269–278.
Hogan B, Beddington R, Costantini F and Lacy E (1994) Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press, New York.
Jänne J, Hyttinen JM, Peura T, Tolvanen M, Alhonen L, Sinervirta R and Halmekytö M (1994) Transgenic bioreactors. Int J Biochem 26(7): 859–870.
Martin MJ, Houtz J, Adams C, Thomas D, Freeman B, Keirns J and Cottrill F (1996) Effect of pronuclear DNA microinjection on the development of porcine ova in utero. Theriogenology 46: 695–701.
Martin MJ and Pinkert CA (1994) Production of transgenic swine. In: Pinkert CA (ed.) Transgenic Animal Technology. (pp. 315–338) Academic Press, San Diego.
Mount LE and Ingram DL (1971) Uses of the pig as a laboratory animal. In: The Pig as a Laboratory Animal. Chap. 11 (pp. 118–125) Academic Press, New York.
Nottle MB, Nagashima H, Verma PJ, Du ZT, Grupen CG, Ashman RJ and MacIlfatrick S (1997) Developments in transgenic techniques in pigs. J Reprod Fert Suppl 52: 237–244.
Pinkert CA, Kooyman DL, Baumgartner A and Keisler DH (1989) In-vitro development of zygotes from superovulated prepubertal and mature gilts. J Reprod Fert 87: 63–66.
Pivko J, Grafenau P, Oberfranc M, Bulla J, Kubovicova E, Laurincik J and Hyttel P (1992) Recovery, structure and viability of porcine zygotes and 2-cell stages subjected to cenrifugation. International Congress on Animal Reproduction and Artifical Insemination, 12: 736–738.
Pursel VG, Hammer RE, Bolt DJ, Palmiter RD and Brinster RL (1990) Integration, expression and germ-line transmission of growth-related genes in pigs. J Reprod Fert Suppl. 41: 77–87.
Pursel VG and Wall RJ (1996) Effects of transferred ova per recipient and dual use of donors as recipients on production of transgenic swine. Theriogenology 46: 201–209.
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB and Erlich HA (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239: 10–19.
Sakimura K, Kushiya E, Takahashi Y and Suzuki Y (1987) The structure and expression of neuron-specific enolase gene. Gene 60: 103–113.
Shimatsu Y, Uchida M, Niki R and Imai H (2000) Induction of superovulation and recovery of fertilized oocytes in prepubertal miniature pigs after treatment with PG600. Theriogenology 53: 1013–1022.
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98: 503–517.
Svendsen O (1998) The minipig in toxicology. Scand J Anim Sci 25 (Suppl. 1).
Swindle MM, Smith AC, Lanber-Laird K and Dungan L (1994) Swine in biomedical research: management and models. ILAR news 36 (1): 1–5.
Swindle MM and Smith AC (1998) Comparative anatomy and physiology of the pig. Scand J Lab Anim Sci 25 (Suppl. 1): 11–21.
Vize PD, Michalska AE, Ashman R, Lloyd B, Stone BA, Quinn P, Wells JRE and Seamark RF (1988) Introduction of a porcine growth hormone fusion gene into transgenic pigs promotes growth. J Cell Science 90: 295–300.
Wagner J, Thiele F and Ganten D (1995) Transgenic animals as models for human disease. Clin Exper Hypertention 17(4): 593–605.
Wall RJ, Pursel VG, Hammer RE and Brinster RL (1985) Development of porcine ova that were centrifuged to permit visualization of pronuclei and nuclei. Biol Reprod 32: 645–651.
Wei LN (1997) Transgenic animals as new approaches in pharmacological studies. Annu Rev Pharmacol Toxicol 37: 119–141.
Wei Q and Chen JFD (1993) Effect of number of transferred microinjected embryos on pregnancy rate and litter size of pigs. Theriogenology vn39: 339.
Willians BL, Sparks AET, Canseco RS, Knight JW, Johnson JL, Velander WH, Page RL, Drohan WN, Kornegay ET, Pearson RE, Wilkins TD and Gwazdauskas FC (1992a) Evaluation of systems for collection of porcine zygotes for DNA microinjection and transfer. Theriogenology 38: 501–511.
Williams BL, Sparks AET, Canseco RS, Knight JW, Johnson JL, Velander WH, Page RL, Drohan WN, Young JM, Pearson RE, Wilkins TD and Gwazdauskas FC (1992b) In vitro development of zygotes from prepubertal gilts after microinjection of DNA. J Anim Sci 70: 2207–2211.
Youngs CR, Ford SP, McGinnis LK and Anderson LH (1993) Investigations into the control of litter size in swine: I. Comparative studies on in vitro development of Meishan and Yorkshire preimplantation embryos. J Anim Sci 71: 1561–1565.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Uchida, M., Shimatsu, Y., Onoe, K. et al. Production of transgenic miniature pigs by pronuclear microinjection. Transgenic Res 10, 577–582 (2001). https://doi.org/10.1023/A:1013059917280
Issue Date:
DOI: https://doi.org/10.1023/A:1013059917280