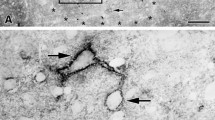
Abstract
The molecular components surrounding a neurone serve as recognition cues for the nerve terminals and glial processes that contact them and the constellations formed by these inputs will therefore be determined by the blend of adhesive and repulsive components therein. Using immunohistochemical methods, we observed that the large extracellular matrix-protein, tenascin-R (Restrictin, J1-160-180, Janusin), associates preferentially with the parvalbumin-positive subpopulation of interneurones within the cerebral cortex. In situ-hybridization indicated that tenascin-R-mRNA was expressed in a subpopulation of nerve cells distinct from that containing parvalbumin, suggesting that this protein's association with the latter is receptor mediated. These nerve cells thus modulate at a distance the composition of the extracellular matrix around parvalbuminneurons.
Similar content being viewed by others
References
Asher, R. & Bignami, A. (1991) Localization of hyaluronate in primary glial cell cultures derived from newborn rat brain. Experimental Cell Research 195, 410–411.
Bartsch, U., Pesheva, P., Raff, M. & Schachner, M. (1993) Expression of janusin (J1–160/180) in the retina and optic nerve of the developing and adult mouse. Glia 9, 57–69.
Bignami, A., Asher, R., Perides, G. & Rahemtulla, F. (1992) The extracellular matrix of cerebral gray matter: Golgi's pericellular net and Nissl's nervöses Grau revisited. International Journal of Developmental Neuroscience 10, 291–299.
Black, J. A. & Waxman, S. G. (1988) The perinodal astrocyte. Glia 1, 169–183.
Brauer, K., Werner, L. & Leibnitz, L. (1982) Perineuronal nets of glia. Journal für Hirnforschung 23, 701–708.
BrÜmmendorf, T., Hubert, M., Treubert, U., Leuschner, R., Tarnok, A. & Rathjen, F.G. (1993) The axonal recognition molecule F11 is a multifunctional protein: Specific domains mediate interactions with Ng-CAM and restrictin. Neuron 10, 711–727.
Celio, M. R. (1986) Parvalbumin in most γ-aminobutyric acid-containing neurons of the rat cerebral cortex. Science 231, 995–997.
Celio, M. R. (1990) Calbindin D-28k and parvalbumin in the rat brain. Neuroscience 35, 375–475.
Celio, M. R. (1993) Perineuronal nets of extracellular matrix around parvalbumin-containing neurons in the hippocampus. Hippocampus 3, 55–60.
Celio, M. R., Baier, W., SchÄrer, L., Viragh, P. A. & Gerday, C. (1988) Monoclonal antibodies directed against the calcium-binding protein parvalbumin. Cell Calcium 9, 81–86.
Celio, M. R. & BlÜmcke, I. (1994) “Perineuronal nets”: A specialized form of extracellular matrix in the adult nervous system. Brain Research Review 19, 128–145.
Chomczynski, P. & Sacchi, N. (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry 162, 156–159.
Endo, T., Kobayashi, M., Kobayashi, S. & Onaya, T. (1986) Immunocytochemical and biochemical localization of parvalbumin in the retina. Cell and Tissue Research 243, 213–217.
Faissner, A. & Kruse, J. (1990) J1/tenascin is a repulsive substrate for central nervous system neurons. Neuron 5, 627–637.
Faissner, A., Kruse, J., Chiquet-Ehrismann, R., & Mackie, E. (1988) The high molecular weight J1 glycoproteins are immunochemically related to tenascin. Differentiation 37, 104–114.
Ffrench-Constant, C., Miller, H. R., Kruse, J., Schachner, M. & Raff, M. C. (1986) Molecular specialization of astrocyte processes at nodes of Ranvier in the rat optic nerve. Journal of Cell Biology 102, 844–852.
Fuss, B., Wintergerst, E. S., Bartsch, U. & Schachner, M. (1993) Molecular characterization and in situ mRNA localization of the neural recognition molecule J1–160–80: A modular structure similar to tenascin. Journal of Cell Biology 120, 1237–1249.
Gennarini, G., Cibelli, G., Rougon, G., Mattei, M. G. & Goridis, C. (1989) The mouse neuronal cell surface glycoprotein F3: A phosphatidyl inositolanchored member of the immunoglobulin superfamily related to chicken contactin. Journal of Cell Biology 109, 775–788.
Golgi, C. (1893) Intorno all'origine del quarto nervo cerebrale e una questione isto-fisiologica che a questo argomento si collega. Rendiconti della reale Accademia dei Lincei (7 maggio). Vol. II, pp. 379–389.
Golgi, C. (1898) Intorno alle strutture delle cellule nervose. Bollettino della Società medico-chirurgica di Pavia. Seduta del 19 aprile: 1–14.
HÄrtig, W., Brauer, K. & BrÜckner, G. (1992) Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. NeuroReport 3, 869–872.
Hendry, S. H. C., Jones, E. G., Hockfield, S. & McKay, R. D. G. (1984) Monoclonal antibody that identifies subsets of neurons in the central visual system of monkey and cat. Nature 307, 267–269.
Hockfield, S. & McKay, R. D. G. (1983) A surface antigen expressed by a subset of neurons in the vertebrate central nervous system. Proceedings of the Natural Academy of Sciences USA 80, 5758–5761.
Kawaguchi, Y., Katsumaru, H., Kosaka, T., Heizmann, C. W. & Hama, K. (1993) Fast spiking cells in rat hippocampus (CA1 region) contain the calcium-binding protein parvalbumin. Brain Research 416, 369–374.
Kawaguchi, Y. & Kubota, Y. (1993) Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin-immunoreactive and calbindin D-28k-immunoreactive neurons in layer V of rat frontal cortex. Journal of Neurophysiology 70, 387–396.
Kosaka, T. & Heizmann, C. W. (1989a) Selective staining of a population of parvalbumin-containing GABAergic neurons in the rat cerebral cortex by lectins with specific affinity for terminal N-acetylgalactosamine. Brain Research 483, 158–163.
Kosaka, T., Heizmann, C. W. & Barnstable, C. J. (1989b) Monoclonal antibody VC1.1 selectively stains a population of GABAergic neurons containing the calcium-binding protein parvalbumin in the rat cerebral cortex. Experimental Brain Research 78, 43–50.
Kosaka, T., Heizmann, C. W. & Fujita, S. C. (1992a) Monoclonal antibody 473 selectively stains a population of GABA-ergic neurons containing the calcium-binding protein parvalbumin in the rat cerebral cortex. Experimental Brain Research 89, 109–114.
Kosaka, T., Heizmann, C. W. & Fujita, S. C. (1992b) Monoclonal antibody 473 selectively stains a population of GABA-ergic neurons containing the calcium-binding protein parvalbumin in the rat cerebral cortex. Experimental Brain Research 89, 109–114.
Kosaka, T., Isogai, K., Barnstable, C. J. & Heizmann, C. W. (1990) Monoclonal antibody HNK-1 selectively stains a subpopulation of GABAergic neurons containing the calcium-binding protein Tenascin-R associates with parvalbumin-interneurones 301 parvalbumin in the rat cerebral cortex. Experimental Brain Research 82, 566–574.
Kruse, J., Keilhauer, G., Faissner, A., Timpl, R. & Schachner, M. (1985) The J1 glycoprotein. A novel nervous system cell adhesion molecule of the L2/HNK1 family. Nature 316, 146–148.
Lafarga, M., Berciano, M. T. & Blanco, M. (1984) The perineuronal net in the fastigial nucleus of the rat cerebellum. Anatomy and Embryology 170, 79–85.
Lochter, A., Taylor, J., Fuss, B. & Schachner, M. (1994) The extracelluilar matrix molecule janusin regulates neuronal morphology in a substrate and culture time dependent manner. European Journal of Neuroscience 6, 597–606.
Lochter, A., Vaughan, L., Kaplony, A., Prochiantz, A., Schachner, M. & Faissner, A. (1991) J1/tenascin in substrate-bound and soluble form displays contrary effects on neurite outgrowth. Journal of Cell Biology 113, 1159–1171.
Lugaro, E. (1895) Sulla struttura del nucleo dentato del cervelletto nell'uomo. Monitore zoologico italiano. 6, 5–12.
LÜth, H. J., Fischer, J. & Celio, M. R. (1992) Soybean lectin binding neurons in visual cortex of the rat contain parvalbumin and are covered by glial nets. Journal of Neurocytology 21, 211–221.
Milev, P., Chiba, A., Haring, M., Rauvala, H., Schachner, M., Ranscht, B., Margolis, R. K. & Margolis, R. U. (1998) High affinity binding and overlapping localization of neurocan and phosphacan/protein-tyrosine phosphatase-zeta/beta with tenascin-R, amphoterin, and the heparinbinding growth-associated molecule. J. Biol. Chem. 273, 6998–7005.
Morganti, M. C., Taylor, J., Pesheva, P. & Schachner, M. (1990) Oligodendrocyte-derived J1–160/180 extracellular matrix glycoproteins are adhesive or repulsive depending on the partner cell type and time of interaction. Experimental Neurology 109, 98–110.
Nagakawa, F., Schulte, B. A., & Spicer, S. S. (1986a) Selective cytochemical demonstration of glycoconjugate-containing terminal N-acetylgalactosamine on some brain neurons. Journal of Comparative Neurology 243, 280–290.
Nagakawa, F., Schulte, B. A., Wu, J. Y. & Spicer, S. S. (1986b) GABA-ergic neurons of the rodent brain correspond partially with those staining for glycoconjugate with N-acetylgalactosamine. Journal of Neurocytology 15, 389–396.
Pesheva, P., Gennarini, G., Goridis, C. & Schachner, M. (1993) The F3/F11 cell adhesion molecule mediates the repulsion of neurons by the extracellular matrix glycoprotein J1–160–180. Neuron 10, 69–82.
Pesheva, P., Spiess, E. & Schachner, M. (1989) J1–160 and J1–180 are oligodendrocyte-secreted, nonpermissive substrates for cell adhesion. Journal of Cell Biology 109, 1765–1778.
RamÓn y Cajal, S. (1898) La red superficial de las células nerviosas centrales. Revista trimestral micrográfica, Madrid 3, 199–206.
Rathjen, F. G., Wolff, J. M. & Chiquetehrismann, R. (1991) Tenascin-R: A chick neural extracellular matrix protein involved in cell attachment co-purifies with the cell recognition molecule F11. Development 113, 151–164.
RÖhrenbeck, J., WÄssle, H. & Boycott, B. B., (1989) Horizontal cells in the monkey retina: Immunocytochemical staining with antibodies against calcium-binding proteins. European Journal of Neuroscience 1, 407–420.
Schachner, M., Taylor, J., Bartsch, U. & Pesheva, P. (1994) The perplexing multifunctionality of janusin, a tenascin-related molecule. Perspectives in Developmental Neurobiology 2, 33–41.
Schwaller, B., Buchwald, P., BlÜmcke, I., Celio, M. R. & Hunziker, W. (1993) Characterization of a polyclonal antiserum against the purified human recombinant calcium-binding protein calretinin. Cell Calcium 14, 601–610.
Srinivasan, J., Schachner, M. & Catterall, W. A. (1998) Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proceedings of the National Academy of Sciences USA 95, 15753–15757.
Streit, W. J., Schulte, B. A., Balentine, J. D. & Spicer, S. S. (1986) Evidence for glycoconjugates in nociceptive primary sensory neurons and its origin from the Golgi-complex. Brain Research 377, 1–17.
Taylor, J., Pesheva, P. & Schachner, M. (1993) The influence of janusin and tenascin on growth cone behavior in vitro. Journal of Neuroscience Research 35, 347–362.
Viggiano, D. (2000) The two faces of perineuronal nets. NeuroReport 14, 2087–2090.
Weber, P., Bartsch, U., Rasband, M. N., Czaniera, R., Lang, Y., Bluethmann, H., Margolis, R. U., Levinson, S. R., Shrager, P., Montag. D. & Schachner, M. (1999) Mice deficient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. Journal of Neuroscience 19, 4245–4262.
Wintergerst, E. S., Fuss, B. & Bartsch, U. (1993) Localization of Janusin mRNA in the central nervous system of the developing and adult mouse. European Journal of Neuroscience 5, 299–310.
Wintergerst, E. S., Vogt-Weisenhorn D., Rathjen F. G., riederer, B. M., Lambert, S. & Celio, M. R. (1996) Temporal and spatial appearance of the membrane cytoskeleton and perineuronal nets in the rat neocortex. Neuroscience Letters 209, 173–176.
Yamamoto, M., Marshall, P., Hemmendinger, L. M., Boyer, A. B. & Caviness, V. S. (1988) Distribution of glucuronic acid-and sulfate containing glycoproteins in the central nervous system of the adult mouse. Neuroscience Research 5, 273–298.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wintergerst, E.S., Rathjen, F.G., Schwaller, B. et al. Tenascin-R associates extracellularly with parvalbumin immunoreactive neurones but is synthesised by another neuronal population in the adult rat cerebral cortex. J Neurocytol 30, 293–301 (2001). https://doi.org/10.1023/A:1014452212067
Issue Date:
DOI: https://doi.org/10.1023/A:1014452212067