Abstract
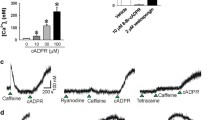
Previous work has shown that stimulation of contraction in A7r5 smooth muscle cells with phorbol ester (PDBu) results in the disassembly and remodeling of the α-actin component of the cytoskeleton (Fultz et al., 2000, J Mus Res Cell Motil 21: 775–781). In the present study, we evaluated the effect of increasing intracellular calcium ion concentration [Ca2+]i by A23187 and thapsigargin on α- and β-actin remodeling. The effects of A23187 and thapsigargin on cell contraction and actin remodeling were effectively identical. The two compounds caused contraction of A7r5 cells that was earlier in onset and more quickly completed than PDBu-induced contractions. Both the α- and β-actin isoforms were incorporated into stress cables in the resting cell. During the interval of contraction, β-actin cables shortened without evidence of disassembly. By comparison, the increase of [Ca2+]i resulted in partial or complete dissolution of α-actin cables without further remodeling. In addition, PDBu-mediated α-actin remodeling was blocked in the presence of A23187. Increased [Ca2+]i also caused dispersal of α-actinin but had no effect on the cellular distribution of talin suggesting the effect was selective for α-actin cytoskeletal structure. The incubation of cells in calcium-free media prevented α-actin dissolution by A23187/thapsigargin and also blocked PDBu-mediated remodeling. Finally, of six kinase inhibitors investigated, only ML-7 partially blocked the dissolution of α-actin cables by increased [Ca2+]i. The results suggest that the sustained elevation of [Ca2+]i beyond a threshold level initiates depolymerization of α-actin but not β-actin. It further appears that PDBu-induced α-actin remodeling requires Ca2+ but increases of [Ca2+]i beyond a threshold level may inhibit this activity. The finding that ML-7 partially inhibits α-actin dissolution in the presence of A23187/thapsigargin may be suggesting that myosin light chain kinase (MLCK) plays a role in destabilizing α-actin structure in the activated cell.
Similar content being viewed by others
References
Battistella-Patterson AS, Wang S and Wright GL (1997) Effect of disruption of the cytoskeleton on smooth muscle contraction. Can J Physiol Pharmacol 75: 1287–1299.
Cross KML, Dahm LM and Bowers CW (2000) Simultaneous measures of contraction and intracellular calcium in single, cultured smooth muscle cells. In Vitro Cell Dev Biol - Animal 36: 50–57.
Ebisawa K, Maruyama K and Nonomura Y (1985) Ca2+ regulation of vertebrate smooth muscle thin filaments mediated by an 84K Mv actin-binding protein: purification and characterization of the protein. Biomed Res 6: 161–173.
Fay FS, Shelvin HH, Granger WC and Taylor SR (1979) Aequorin luminescence during activation of single isolated smooth muscle cells. Nature 280: 506–508.
Fultz ME, Li C, Geng W and Wright GL (2000) Remodeling in the contracting A7r5 smooth muscle cell. J Muscle Res Cell Motil 21: 775–787.
Grynkiewicz G, Poenie M and Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260(6): 3440–3450.
Gunst SJ, Wu MF and Smith DD (1993) Contraction history modulates isotonic shortening velocity in smooth muscle. Am J Physiol 265(34): C467–C476.
Harris DE and Warshaw DM (1990) Slowing of velocity during isotonic shortening in isolated smooth muscle cells. J Gen Physiol 96: 581–601.
Harris DE and Warshaw DM (1991) Length vs. active force relationship in single isolated smooth muscle cells. Am J Physiol 260(29): C1104–C1112.
Himpens B and Casteels R (1990) Different effects of depolarization and muscarinic stimulation on the Ca2+/force relationship during the contraction/relaxation cycle in the guinea-pig ileum. Pflügers Archiv 416: 28–35.
Himpens B, Matthÿs G, Somlyo AV, Butler TM and Somlyo AP (1988) Cytoplasmic free calcium, myosin light chain phosphorylation, and force in phasic and tonic smooth muscle. J Gen Physiol 92: 713–729.
Ishü N, Simpson AW and Ashley CC (1989) Free calcium at rest during ‘catch’ in single smooth muscle cells. Science 243(4896): 1367–1368.
Itoh H and Lederis K (1987) Contraction of rat thoracic aorta strips induced by phorbol-12-myristate-13-acetáte. Am J Physiol 252: C244–C247.
Jiang MJ and Morgan KG (1987) Intracellular calcium levels in phorbol-ester-induced contractions of vascular smooth muscle. Am J Physiol 253: H1365–H1371.
Karreda T, Shimizu K, Nakaiyo S and Urakawa N (1995) Effect of phorbol ester, 12-deoxyphorbol-13-isobutylate (DPB), on muscle tension and cytosolic Ca2+ in rat anococcygeus muscle. Jpn J Pharmacol 69: 195–204.
Kwiatkowski DJ (1999) Functions of gelsolin: motility, signaling, apoptosis, cancer. Curr Opin Cell Biol 11: 103–108.
Mehta D and Gunst SJ (1999) Actin polymerization stimulated by contractile activation regulates force development in canine tracheal smooth muscle. J Physiol (Lond) 519(3): 829–840.
Miyauchi Y, Oishi K and Uchida MK (1994) Actin severing and Ca2+-induced reversal of smooth muscle contraction that is independent of Ca2+. Gen Pharmacol 25(4): 691–695.
Morgan JP and Morgan KG (1982) Vascular smooth muscle: the first recorded Ca2+ transients. Pflugers Arch 395(1): 75–77.
Morgan JP and Morgan KG (1984) Stimulus-specific patterns of intracellular calcium levels in smooth muscle of the ferret portal vein. J Physiol (Lond) 351: 155–167.
Murthy KS, Yee YS, Grider JR and Makhlouf GM (2000) Phorbolstimulated Ca2+ mobilization and contraction in dispersed intestinal smooth muscle cells. J Pharmacol Exp Ther 294(3): 991–996.
Nakajima S, Fujimoto M and Veda M (1993) Spatial changes of [Ca2+]i and contraction by phorbol esters in vascular smooth muscle cells. Am J Physiol 265: C1138–C1145.
Ozaki H, Sato K, Sakata K and Karaki H (1989) Endothelin dissociates muscle tension from cytosolic Ca2+ in vascular smooth muscle of rat carotid artery. Jpn J Pharmacol 50: 521–524.
Rembold CM and Murphy RA (1986) Myoplasmic calcium, myosin phosphorylation and regulation of the crossbridge cycle in swine arterial smooth muscle. Circ Res 58: 803–815.
Rembold CM and Murphy RA (1988) [Ca2+]i-dependent myosin phosphorylation in phorbol-diester-stimulated smooth muscle contraction. Am J Physiol 255: C719–C723.
Rhoten WB and Sergeev IN (1994) Calbindin-D28K appears to buffer intracellular Ca2+ in butyrate-treated rat insulinoma cell line. Endocrine 2: 989–995.
Shen X, Wu MF, Tepper RS and Gunst SJ (1997) Mechanisms for mechanical response of airway smooth muscle to length oscillation. J Appl Physiol 83(3): 731–738.
Singer HA and Baker KM (1987) Calcium dependence of phorbol-12,13-dibutyrate-induced force and myosin light chain phosphorylation in arterial smooth muscle. J Pharmacol Exp Ther 243(3): 814–821.
Small JV, Furst DO and DeMeys L (1986) Localization of filamin in smooth muscle. J Cell Biol 102: 210–220.
Somlyo AP (1985) Excitation-contraction coupling and the ultrastructure of smooth muscle. Circ Res 57: 497–501.
Sybertz EJ, Desiderio DM, Tetzlo. G and Chiu PJS (1986) Phorbol dibutyrate contraction in rabbit aorta: calcium dependence and sensitivity to nitrovasodilators and 8-BR-cyclic GMP. J Pharmacol Exp Ther 239: 78–83.
Theler JM, Mollard P, Guerineau N, Vaches P, Pralong WF, Schlegel W and Wollheim CB (1992) Video imaging of cytosolic Ca2+ in pancreatic β-cells stimulated by glucose, carbacol and ATP. J Biol Chem 267(25): 18, 110-18, 117.
Wright GL and Battistella-Patterson AS (1998) Involvement of the cytoskeleton in calcium-dependent stress relaxation of rat aortic smooth muscle. J Mus Res Cell Motil 19: 405–414.
Wright G and Hurn E (1994) Cytochalasin inhibition of slow tension increase in rat aortic rings. Am J Physiol 267(36): H1437–H1447.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, C., Fultz, M., Parkash, J. et al. Ca2+-dependent actin remodeling in the contracting A7r5 cell. J Muscle Res Cell Motil 22, 521–534 (2001). https://doi.org/10.1023/A:1015026530258
Issue Date:
DOI: https://doi.org/10.1023/A:1015026530258