Abstract
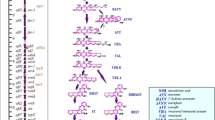
The relationship between the activities of 3 cytosolic enzymes with aflatoxin biosynthesis in Aspergillus parasiticus cultured under different conditions has been investigated in order to find out the role of each enzyme in aflatoxin biosynthesis. Basically the activity of isocitrate dehydrogenase (IDH) was higher in non-toxigenic strains as compared to its counterpart toxigenic fungi (p < 0.05). In contrast, the activities of fatty acid synthase (FAS) as well as glutathione S-transferase (GST) were higher (P < 0.05) in toxigenic strains than that of the non-toxigenic fungi. Aflatoxin production was inhibited in fungi grown in presence of various concentrations of neem leaf extract. Aflatoxin was at its lowest level (>90% inhibition) when the concentration of neem extract was adjusted to 50% (v/v). No significant changes in FAS and IDH activities were observed when aflatoxin synthesis was under restraints by neem (Azadirachta indica) leaf extract. During a certain period of time of culture growth, when aflatoxin production reached to its maximum level, the activity of FAS was slightly induced in the toxigenic strains fed with a low concentration (1.56% v/v) of the neem leaf extract. At the time (96 h) when aflatoxin concentration reached to its maximum levels, the activity of GST in the toxigenic fungi was significantly higher (i.e., 7–11 folds) than that of non-toxigenic strains. The difference was highest in mycelial samples collected after 120 h. However unlike FAS and IDH, GST was readily inhibited (∼67%) in mycelia fed with 1.56% v/v of the neem extract. The inhibition reached to maximum of 80% in samples exposed to 6.25–12.5% of the extract. These results further substantiate previous finding that there is a positive correlation between GST activity and aflatoxin production in fungi.
Similar content being viewed by others
References
Khan M, Schneider B, Wassilew SW, Splanemann V. Experimental study of the effect of raw materials of the neem tree and neem extracts on dermatophytes, yeast and molds. Z Hautkr 1988; 15; 63: 499–502.
Badman L, Joshi SP, Bedekar SS. In vitro antiviral activity of neem (Azadirachta indica, A. Juss) leaf extract against coxsachieviruses. J Commun Dis 1999; 31: 79–90.
SaiRam M, Ilavazhagan G, Sharma SK, Dhanraj SA, Suresh B, Panda M, Devendra K, Selvamurthy W. Anti-microbial activity of a vaginal contraceptive NIM-76 and neem oil (Azadirachta indica). J Ethnopharmacol 2000; 71: 377–382.
Schaaf O, Jarvis AP, Van der Esch SA, Giagnacovo G, Oldham NJ. Rapid and sensitive analysis of azadirachtin and related triterpenoids from neem (Azadirachta indica) by high performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J Chromatogr A 2000; 886: 89–97.
Siddiqui BS, Afshan F, Ghiasuddin, Faizi S, Naqvi SN, Tariq RM. Two insecticidal tetranorcidals from Azadirachta indica. Phytochemistry 2000; 53: 371–371.
Siddiqi S, Faizi S, Siddiqui BS, Ghiasuddin. Constituents of Azadirachta indica: Isolation and structure elucidation of a new antibacterial tetranortriterpenoid, mahmoodin, and a new protolimonoid, naheedin. J Nat Prod 1992; 55: 303–310.
Bhatnagar D, McCormic SP. The inhibitory effect of neem (Azadirachta indica) leaf extracts on aflatoxin synthesis in Aspergillus parasiticus. J Am Oil Chem Soc 1988; 65: 1166–1168.
Zeringue HJ Jr, Bhatnagar D. Inhibition of aflatoxin production in Aspergillus flavus infected cotton bolls after treatment with neem (Azadirachta indica) leaf extracts. J Am Oil Chem Soc 1990; 67: 215–216.
Eaton DJ, Groopman JD. The toxicology of aflatoxins. Academic Press, New York, 1993.
Gupta SK, Maggon KK, Venkitasubramanian TA. Effect of zinc on tricarboxylic acid cycle intermediates and enzymes in relation to aflatoxin biosynthesis. J Gen Microb 1977; 99: 43–48.
Shantha T, Murthy VS. Influence of tricarboxylic acid cycles intermediates and related metabolites on the biosynthesis of aflatoxin by resting cells of Aspergillus flavus. Appl Envirn Microbiol 1981; 42: 758–761.
Mahanti N, Bhatnagar D, Cary JW, Joubran J, Linz JE. Structure and function of fas-1A, a gene encoding a putative fatty acid synthethase directly involved in aflatoxin biosynthesis in A. parasiticus. Appl Environ Microbiol 1996; 62: 191–195.
Buchanan RL, Jones SB, Gerasimowicz WV, Zaica LL, Stahl HG, Ocker LA. Regulation of aflatoxin biosynthesis: Assessment of the role of cellular energy status as a regulator of the induction of aflatoxin production. Appl Envirn Microbiol 1987; 53: 1224–1231.
Davis ND, Diener UL, Eldridge DW. Production of aflatoxins B1 and G1 by Aspergillus flavus in semisynthetic medium. Appl Microbiology 1966; 14: 378–380.
Nabney J, Nesbitt, BF. A spectrophotometric method for determining aflatoxins. Analyst 1965; 90: 155–160.
Meixner-Monori B, Kubicek CP, Harrer W, Schreferl G, Rohr M. NADPH-specific isocitrate dehydrogenase from the citricaccumulating fungus Aspergillus niger. Biochem J 1986; 236: 549–557.
Lynen F. Fatty acid synthesis from malonyl CoA. In: Methods in Enzymology, vol. 5, Academic Press, New York, 1962, 443–451.
Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J Biol Chem 1974; 249: 7130–7139.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 1976; 27: 248–254.
Brian PW, Dawkins AW, Grove JF, Hemming HG, Lowe D, Norris GLF. Phytotoxic compounds produced by Fusarium Equiset. J Expt Bot 1961; 12: 1–12.
Yu J, Woloshuk CP, Bhatnagar D, Cleveland TE. Cloning and characterization of avfa and omtB genes involved in aflatoxin biosynthesis in three Aspergillus species. Gene 2000; 248: 157–167.
Yu J, Chang PK, Ehrlich KC, Cary JW, Montalbano B, Dyer JM, Bhatnagar D, Cleveland TE. Characterization of the critical amino acids of an Aspergillus parasiticus cytochrome P-450 monooxygenase encoded by ordA that is involved in the biosynthesis of aflatoxins B1, B2, and G2. Appl Environ Microbiol 1998; 64: 4834–4841.
Yu J, Chang PK, Cary JW, Bhatnagar D, Cleveland TE. avn A, a gene encoding a cytochrome P-450 monooxygenase, is involved in the conversion of averantin to averufin in aflatoxin biosynthesis in Aspergillus parasiticus. Appl Environ Microbiol 1977; 63: 1349–1356.
Woloshuk CP, Prieto R. Genetic organization and function of the aflatoxin B1 biosynthetic genes. FEMS Microbiol Lett 1998; 169: 169–176.
Saxena M, Mukerji KG, Raj HG. Positive correlation exists between glutathione S-transferase activity and aflatoxin formation in A. flavus. Biochem J 1988; 254: 567–570.
Neal GE, Metcalfe SA, Legg RF, Judah DJ, Green JA. Mechanism of the resistance of cytotoxicity with proceeds in aflatoxin B1 hepatocarcinogenesis. Carcinogenesis 1981; 2: 457–461.
Saxena M, Allameh A, Raj HG, Mukerji KG. Studies on glutathione S-transferases of A. flavus group in relation to aflatoxin production. J Toxicology-Toxin Rev 1989; 8: 319–328.
Saxena M, Allameh A, Mukerji KG, Raj HG. Epoxidation of aflatoxin B1 by Aspergillus flavus microsomes in vitro: Interaction with DNA and formation of AFB1-glutathione conjugate. Chemico-Biol Interactions 1991; 78: 13–22.
Degan DH, Neumann HG. The major metabolite of aflatoxin B1 in the rat is a glutathione conjugate. Chemico-Biol Interactions 1978; 22: 239–255.
Raj HG, Lotlikar PD. Urinary excretion of thiol conjugates of aflatoxin B1 in rats and hamsters. Cancer Lett 1984; 22: 125–133.
Bennett JW, Christensen SW. New perspectives on aflatoxin biosynthesis. Adv Appl Microbiol 1983; 29: 53–92.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Allameh, A., Abyaneh, M.R., Shams, M.R. et al. Effects of neem leaf extract on production of aflatoxins and activities of fatty acid synthetase, isocitrate dehydrogenase and glutathione S-transferase in Aspergillus parasiticus . Mycopathologia 154, 79–84 (2002). https://doi.org/10.1023/A:1015550323749
Issue Date:
DOI: https://doi.org/10.1023/A:1015550323749