Abstract
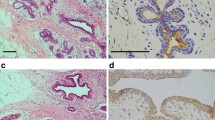
Annexins are a family of structurally related, water-soluble proteins that have calcium- and phospholipid-binding domains. Annexin I is thought to be involved in cell proliferation and differentiation and has recently been shown to be expressed on the surfaces of lymphoma cells where it acts as an endothelial cell adhesion molecule. To evaluate the expression of annexin I in relation to human breast cancer development and progression we used breast biopsy tissues. Immunohistochemical analysis of annexin I in paraffin-embedded ductal epithelial cells of various human breast tissues indicated that this annexin was not demonstrable in the ductal luminal cells of normal breast tissues (n = 11) and benign tumors (n = 10) (except for one ductal adenoma) but was generally expressed in various types of breast cancers, including noninvasive ductal carcinoma in situ (DCIS), invasive and metastatic breast tumors (n = 33). The results suggest that annexin I expression might correlate with malignant breast cancer progression but it is most likely involved at an early stage of human breast cancer development.
Similar content being viewed by others
References
Crumpton MJ and Dedman JR, 1990, Protein terminology tangle. Nature, 345, 212.
Crompton MR, Moss SE and Crumpton MJ, 1988, Diversity in the lipocortin/calpactin family. Cell, 55, 1–3.
Pepinsky RB, Tizard R, Mattaliano RJ, et al. 1988, Five distinct calcium and phospholipid binding proteins share homology with lipocortin I. J Biol Chem, 263, 10799–811.
Ernst JD, Mall A and Chew G, 1994, Annexins possess functionally distinguishable Ca2+ and phospholipid binding domains. Biochem BiophysRes Commun, 200, 867–76.
Burgoyne RD and Geisow MJ, 1989, The annexin family of calcium-binding proteins. Cell Calcium, 10, 1–10.
Hoekstra D, Buist-Arkema R, Klappe K and Reutelingsperger CP, 1993, Interaction of annexins with membranes: the N-terminus as a governing parameter as revealed with a chimeric annexin. Biochemistry, 32, 14194–202.
Klee CB, 1988, Calcium dependent phospholipid and membrane binding proteins. Biochemistry, 27, 6645–53.
Futter CE, Felder S, Schlessinger J, Ullrich A and Hopkins CR, 1993, Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J Cell Biol, 120, 77–83.
Oudinet JP, Russomarie F, Cavadore JC and Rothhut B, 1993, Protein kinase C-dependent phosphorylation of annexins I and II in mesangeal cells. Biochem J, 292, 63–8.
Michener ML, Dawson WB and Creutz CE, 1986, Phosphorylation of a chromaffin granule-binding protein in stimulated chromaffin cells. J Biol Chem, 261, 6548–55.
Masaki T, Tokuda M, Fujimura T, et al. 1994, Involvement of annexin I and annexin II in hepatocyte proliferation: can annexins I and II be markers for proliferative hepatocytes? Hepatology, 20, 425–35.
Wong WT, Nick HS and Forst SC, 1992, Regulation of annexin I in adipogenesis: cAMP-independent action of methylisobutylxanthine. Am J Physiol, 262, 91–7.
Lozano JJ, Siberstein GB, Hwang SI, Haindl AH and Rocha V, 1989, Developmental regulation of calcium binding proteins (calelectrins and calpactin I) in mammary gland. J Cell Physiol, 138, 503–10.
Horlick KR, Ganjianpour M, Frost SC and Nick HS, 1991, Annexin I regulation in response to suckling and rat mammary cell differentiation. Endocrinology, 128, 1574–9.
Creutz CE, Kambouris NG, Snyder SL, et al. 1992, Effects of the expression of mammalian annexins in yeast secretory mutants. J Cell Sci, 103, 1177–92.
Raynal P, Van Bergen en Henegouwen PMP, Hullin F, et al. 1992, Morphological and biochemical evidence for partial nuclear localization of annexin I in endothelial cells. Biochem Biophys Res Commun, 186, 432–9.
Naritoku WY and Taylor CR, 1988, A comparative study of the use of monoclonal antibodies using three different immunohistochemical methods. J Histochem Cytochem, 30, 253–60.
Zokas L and Glenney Jr JR, 1987, The calpactin light chain is tightly linked to the cytoskeleton form of calpactin I; studies using monoclonal antibodies to calpactin subunits. J Cell Biol, 105, 2111–20.
Zokas L, 1988, Antibodies to the N-terminus of calpactin I affect Ca+2 binding and phosphorylation by the epidermal growth factor receptor In vitro. Biochemistry, 27, 2069–76.
Woods GS and Warnke R, 1981, Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection system. J Histochem Cytochem, 29, 1196–204.
Wong WT, Frost SC and Nick HS, 1991, Proteinsynthesis-dependent induction of annexin I by glucocorticoid. Biochem J, 275, 313–9.
Violette SM, King I, Browning JL, Pepinsky RB, Wallner BP and Sartorelli AC, 1990, Role of lipocortin I in the glucocorticoid induction of the terminal differentiation of a human squamous carcinoma. J Cell Physiol, 142, 70–7.
Pencil SD, Toh Y and Nicolson GL, 1993, Candidate metastasis-associated genes of the rat 13762NF mammary adenocarcinoma. Breast Cancer Res Treatm, 25, 165–74.
Toh Y, Pencil SD and Nicolson GL, 1994, A novel candidate metastasis-associated gene mta1differentially expressed in highly metastatic mammary adenocarcinoma cell lines: cDNA cloning, expression and protein analyses. J Biol Chem, 269, 22958–63.
Yeatman TJ, Updyke TV, Kaetzel MA, Dedman JR and Nicolson GL, 1993, Expression of annexins on the surfaces of non-metastatic and metastatic human and rodent tumor cells. Clin Exp Metastasis, 11, 37–44.
Nicolson GL, 1991, Quantitative variations in gene expression: possible role in cellular diversification and tumor progression. J Cell Biochem, 46, 277–83.
Nicolson GL, 1991, Gene expression and tumor progression to the metastatic phenotype. Bioessays, 13, 337–42.
Tressler RJ, Updyke TV, Yeatman TJ and Nicolson GL, 1993, Extracellular annexin II is associated with divalent cation-dependent tumor cell-endothelial cell adhesion of metastatic RAW 117 large-cell lymphoma cells. J Cell Biochem, 53, 265–76.
Tressler RJ, Updyke TV, Yeatman T and Nicolson GL, 1994, Extracellular annexin VI is associated with divalent cation-dependent endothelial cell adhesion of metastatic RAW117 large-cell lymphoma cells. Exp Cell Res, 215, 395–400.
Schwartz-Albiez R, Koretz K, Moller P and Wirl G, 1993, Differential expression of annexins I and II in normal and malignant human mammary epithelial cells. Differentiation, 52, 229–37. 1
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ahn, SH., Sawada, H., Ro, JY. et al. Differential expression of annexin I in human mammary ductal epithelial cells in normal and benign and malignant breast tissues. Clin Exp Metastasis 15, 151–156 (1997). https://doi.org/10.1023/A:1018452810915
Issue Date:
DOI: https://doi.org/10.1023/A:1018452810915