Abstract
Purpose. To develop an integrated absorption model for estimating the fraction of dose absorbed and determining the causes of poor oral drug absorption.
Methods. Both analytical and numerical methods were used to estimate the fraction of dose absorbed.
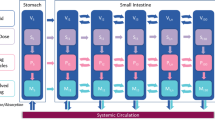
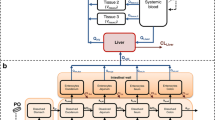
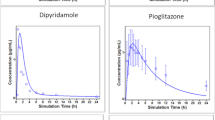
Results. An integrated absorption model was developed by considering transit flow, dissolution, and permeation processes, simultaneously. A framework was proposed to determine permeability-, dissolution-, and solubility-limited absorption. Digoxin, griseofulvin, and panadiplon were employed to illustrate the applications of the integrated model in identifying the causes of poor absorption and guiding formulation development.
Conclusions. The integrated absorption model was successfully applied to digoxin, griseofulvin, and panadiplon to estimate the fraction dose absorbed and to roughly determine the causes of poor oral drug absorption.
Similar content being viewed by others
REFERENCES
L. X. Yu, J. R. Crison, E. Lipka, and G. L. Amidon. Transport approaches to the biopharmaceutical design of oral drug delivery systems: prediction of intestinal absorption. Adv. Drug Del. Rev. 19:359-376 (1996).
L. X. Yu and G. L. Amidon. Characterization of small intestinal transit time distribution in humans. Int. J. Pharm. 171:157-163 (1998).
L. X. Yu and G. L. Amidon. Saturable small intestinal drug absorption in humans: modeling and explanation of the cefatrizine data. Eur. J. Pharm. Biopharm. 45:199-203, 1998.
M. Mayersohn. Principles of drug absorption. In G. S. Banker and C. T. Rhodes (eds.), Modern Pharmaceutics, 2nd ed., Marcel Dekker: New York, 1990, pp. 23-90.
R. J. Hintz and K. C. Johnson. The effect of particle size distribution on the dissolution rate and oral absorption. Int. J. Pharm. 51:9-17, 1989.
K. A. Kelly. In L. R. Johnson (Editor-in-Chief), Physiology of the Gastrointestinal Tract, Vol 1, Raven Press, New York, 1981, pp. 393-410.
J. B. Dressman and D. Fleisher. Mixing-tank model for predicting dissolution rate control of oral absorption. J. Pharm. Sci. 75:109-116 (1986).
G. L. Amidon, H. Lennernas, V. P. Shah, and J. R. Crison. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12:413-420 (1995).
D. Z. D'Argenio and A. Schumitzky. Adapt II: Mathematical Software for Pharmacokinetic/Pharmacodynamic Systems Analysis, University of Southern California, Los Angles, 1992.
A. Astrorri, G. Bianchi, G. LaCanna, D. Assanelli, O. Visioli, and A. Marzo. Bioavailability and related heart function index of digoxin capsules and tablets in cardiac patients. J. Pharm. Sci. 68:104-106 (1979).
B. F. Johnson, J. O'Grady, and C. Bye. The influence of digoxin particle size on absorption of digoxin and the effect of porpantheline and metoclopramide. Br. J. Clin. Pharmac. 5:465-467 (1978).
A. J. Jounela, P. J. Pentikainen, and A. Sothmann. Effect of particle size on the bioavailability of digoxin. Eur. J. Clin. Pharmacol. 8:365-370 (1995).
T. R. D. Shaw and J. E. Carless. The effect of particle size on the absorption of digoxin. Eur. J. Clin. Pharmacol. 7:269-273 (1974).
D. H. Huffman and D. L. Azarnoff. Absorption of orally given digoxin preparations. JAMA. 222:957-960 (1972).
V. Manninen, A. Apajalahti, J. Melin, and M. Karesoja. Altered absorption of digoxin in patients given propantheline and metoclopramide. Lancet, 398-400 (1973).
T. Gramatte. Griseofulvin absorption from different sites in the human small intestine. Biopharm. Drug Dis. 15:747-759 (1994).
W. L. Chiou and S. Riegelman. Absorption characteristics of solid dispersed and micronized griseofulvin in man. J. Pharm. Sci. 60:1376-1380 (1971).
P. Kabasakalian, M. Katz, B. Rosenkrantz, and E. Townley. Parameters affecting absorption of griseofulvin in a human study using urinary metabolite excretion data. J. Pharm. Sci. 59:595-600 (1970).
T. Nishihata, M. Ishizaka, S. Yokohama, A. C. Martino, and R. E. Gordon. Effects of particle size of bulk drug and food on the bioavailability of U-78875. Drug Dev. Ind. Pharm. 19:2679-2698 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, L.X. An Integrated Model for Determining Causes of Poor Oral Drug Absorption. Pharm Res 16, 1883–1887 (1999). https://doi.org/10.1023/A:1018911728161
Issue Date:
DOI: https://doi.org/10.1023/A:1018911728161