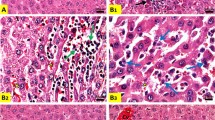
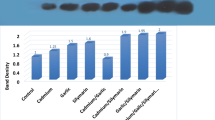
Abstract
The effect of cisplatin on five glutathione-related enzymes was studied in liver, kidney, and Dalton lymphoma cells of tumor-bearing mice. In liver, the activities of glutathione S-transferase, glutathione peroxidase, catalase, and superoxide dismutase decreased approximately 30–40%, 60–67%, 35–50% and 70–80% respectively, while glutathione reductase increased about 36–45% after cisplatin treatment. In kidney, catalase activity decreased by 47–82% at all time points (24–96 h) of cisplatin treatment, while glutathione S-transferase activity decreased significantly (~24%) mainly at 72 h of treatment. An increase in glutathione reductase (~1.5–2.5 times), glutathione peroxidase (significant at 24 h, 47%), and superoxide dismutase (~15–60%) was noted in kidney after the treatment. In Dalton lymphoma cells, the activities of glutathione S-transferase, glutathione peroxidase, and catalase decreased very distinctly (~2–5, 2–5 and 5–11 times, respectively) at all time points, but glutathione reductase decreased significantly only at 72 h of cisplatin treatment. Interestingly, the superoxide dismutase activity in Dalton lymphoma cells increased initially at 24–48 h and then decreased (~60%) during later periods (72–96 h) of treatment. Cisplatin treatment caused a decrease in glutathione level in Dalton lymphoma cells (~14–20%) and kidney (~18–28%) but no change in liver. In view of the results, a definite correlation with the changes in glutathione concentrations and enzymatic activities in a tissue could not be firmly derived. It is suggested that the changes in various glutathione-related enzymes and glutathione levels in the tissues of the host during cisplatin-mediated chemotherapy could affect cellular antioxidant defense potential, which may play an important contributory role in cisplatin-mediated toxicity, particularly nephrotoxicity, and anticancer activity in the host.
Similar content being viewed by others
References
Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121-6.
Arruda VR, Salles TSI, Costa FF, Sead STO. Glutathione peroxidase, reduced glutathione, superoxide dismutase and catalase in red cells of patient with hairy cells leukemia. Neoplasma. 1996;43:99-102.
Black SM, Wolf CR. The role of glutathione-dependent en-zymes in drug resistance. Pharmacol Ther. 1991;51:139-54.
Canada AT, Herman L, Kidd K, Robertson C, Trump D. Glutathione depletion increases the cytotoxicity of melpha-lan to the androgen-insensitive prostate cancer cell lines. Cancer Chemother Pharmacol. 1993;32:73-7.
Chien C, Kirollos KS, Linderman RJ, Dauterman WC. α,β-Unsaturated carbonyl compounds: inhibition of rat liver glutathione S-transferase isozymes and chemical reaction with reduced glutathione. Biochim Biophys Acta. 1994; 1204:175-80.
Collins JL, Kao M. The anticancer drug cisplatin increases the naturally occuring cell-mediated lysis of tumor cells. Cancer Immunol Immunother. 1989;29:17-22.
Corrocher R, Casaril M, Bellisola G, et al. Severe impairment of antioxidant system in human hepatoma. Cancer. 1986; 58:1658-62.
Coste F, Malinge J, Serra L, et al. Crystal structure of a double-stranded DNA containing a cisplatin intrastrand cross-link at 1.63 Åresolution-hydration at the platinated site. Nu-cleic Acids Res. 1999; 27: 1837-46.
Deleve LG, Kaplowitz N. Glutathione metabolism and its role in hepatotoxicity. Pharmacol Ther. 1991;52:287-305.
Flohe L, Gunzler WA. Assay of glutathione peroxidase. Meth-ods Enzymol. 1984;105:114-21.
Flohe L, Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93-104.
Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97-112.
Giri A, Khynriam D, Prasad SB. Vitamin C mediated protec-tion on cisplatin induced mutagenicity in mice. Mutation Res. 1998a;421:139-48.
Giri A, Khynriam D, Prasad SB. Use of vitamin C against cisplatin induced mutagenicity and nephrotoxicity. In: Shar-an R, ed, Trends in radiation and cancer biology. Vol. 29. Germany: Forschungszentrum Julich; 1998b:166-76.
Go RS, Adjei AA. Review of comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999;17:409-22.
Gromadzinska J, Wasowicz W, Andrijewski M, et al. Glu-tathione and glutathione metabolizing enzymes in tissues and blood of breast cancer patients. Neoplasma. 1997;44: 45-51.
Habig WH, Pabot MJ, Jarkoby WB. Glutathione S-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130-9.
Hansson J, Berhane K, Castro VM, Jungnelius U, Mannervik B, Ringborg U. Sensitization of human melanoma cells to the cytotoxic effect of melphalan by the gutathione transfer-ase inhibitor ethacrynic acid. Cancer Res. 1991;51:94-8.
Hayes JD, Pulford DJ. The glutathione S-transferase super gene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445-600.
Ishikawa T, Ali-Osman F. Glutathione-associated cis-diammi-nedichloroplatinum(II) metabolism and ATP-dependent ef-flux from leukemia cells: molecular characterization of glutathione platinum complex and its biological signifi-cance. J Biol Chem. 1993;268:20116-25.
Jones MM, Basinger MA. Thiol and thioether suppression of cis-platinum-induced nephrotoxicity in rats bearing the Walker 256 carcinoma. Anticancer Res. 1989;9:1937-42.
Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res. 2001;478:23-43.
Kharbangar A, Khynriam D, Prasad SB. Effect of cisplatin on mitochondrial protein, glutathione and succinate dehydro-genase activity. Cell Biol Toxicol. 2000;16:363-73.
Khynriam D, Prasad SB. Hematotoxicity and blood glutathione levels after cisplatin treatment of tumor-bearing mice. Cell Biol Toxicol. 2001;17:357-70.
Kodera Y, Isobe K, Yamauchi M, et al. Expression of glu-tathione S-transferases α and π in gastric cancer: a correla-tion with cisplatin resistance. Cancer Chemother Pharmacol. 1994;34:203-8.
Krakoff IH. Nephrotoxicity of cis-dichlorodiammineplatinum. Cancer Treat Rep. 1979;63:1523-5.
Lowry OH, Rosebrough NJ, Farr AL, Randal RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-75.
Meister A. Glutathione deficiency produced by inhibition of its synthesis and its reversal: application in research and therapy. Pharmacol Ther. 1991;51:155-94.
Nakano S, Gemba M. Potentiation of cisplatin-induced lipid peroxidation in kidney cortical slices by glutathione deple-tion. Jpn J Pharmacol. 1989;50:87-92.
Navarro J, Obrador E, Carretero J, et al. Changes in glu-tathione status and the antioxidant system in blood and in cancer cells associate with tumour growth in vivo. Free Radic Biol Med. 1999;26:410-18.
Ohkuwa T, Sato Y, Naoi M. Glutathione status and reactive oxygen generation in tissues of young and old exercised rats. Acta Physiol Scand. 1997;159:237-44.
Paoletti F, Mocali A. Determination of superoxide dismutase activity by purely chemical system based on NAD(P)H oxidation. Methods Enzymol. 1990;186:209-21.
Prasad SB, Giri A. Antitumour effect of cisplatin against murine ascites Dalton's lymphoma. Indian J Exp Biol. 1994;32:155-62.
Prasad SB, Giri A. Cisplatin-induced changes in tissue calcium and potassium concentrations in tumour-bearing mice. Med Sci Res. 1999;27:459-62.
Prasad SB, Sodhi A. Effect of cis-dichlorodiammineplatinu-m(II) on the agglutinability of tumor and normal cells with concanavalin A and wheat germ agglutinin. Chem Biol Interact. 1981;36:355-67.
Prasad SB, Giri A, Khynriam D, Kharbangar A, Nicol BM, Lotha C. Cisplatin-mediated enzymatic changes in mice bearing ascites Dalton's lymphoma. Med Sci Res. 1999;27: 723-30.
Rosenberg B. Fundamental studies with cisplatin. Cancer. 1985;55:2303-16.
Sadzuka Y, Shoji T, Takino Y. Change of lipid peroxide levels in rat tissues after cisplatin administration. Toxicol Lett. 1991; 57:159-66.
Sedlak J, Lindsay RH. Estimation of total, protein-bound and non-protein sulfhydryl groups in tissue with Ellman's re-agent. Anal Biochem. 1968;25:192-205.
Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916-21.
Slater TF. Free radical mechanism in tissue injury. Biochem J. 1984;222:1-15.
Smith IK, VierHeller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenate using 5,5'-dithio-bis(2-nitrobenzoic acid). Anal Biochem. 1988;175:408-13.
Sweet WL, Blanchard JS. Human erythrocyte glutathione reductase: chemical mechanism and structure of the transi-tion state for hydride transfer. Biochem. 1991;30:8702-9.
Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991; 51:794-8.
Teramato S, Tomita T, Matsui H, Ohga E, Matsuse T, Ouchi Y. Hydrogen peroxide-induced apoptosis and necrosis in hu-man lung fibroblasts: protective roles of glutathione. Jpn J Pharmacol. 1999;79:33-40.
Tew KD. Glutathione associated enzymes in anticancer drug resistance. Cancer Res. 1994;54: 4313-20.
Tew KD, Bomber AM, Hoffman, SJ. Ethacrynic acid and piriprost as enhancers of cytotoxicity in drug resistant and sensitive cell lines. Cancer Res. 1988;48:3622-5.
Ueda M, Mozaffar S, Tanaka A. Catalase from Candida boidini 2201. Methods Enzymol. 1990;188:463-7.
Wang W, Ballatori N. Endogenous glutathione conjugates: occurrence and biological functions. Pharmacol Rev. 1998;50:335-55.
Zwelling LA, Anderson T, Kohn KW. DNA-protien and DNA inter-strand cross-linking by cis-and trans-platinum(II) diamminedichloride in 1210 mouse leukemia cells and relation to cytotoxicity. Cancer Res. 1979;39:365-9.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Khynriam, D., Prasad, S. Changes in glutathione-related enzymes in tumor-bearing mice after cisplatin treatment. Cell Biol Toxicol 18, 349–358 (2002). https://doi.org/10.1023/A:1020899221192
Issue Date:
DOI: https://doi.org/10.1023/A:1020899221192