Abstract
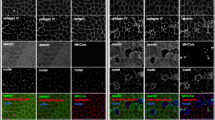
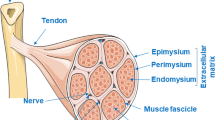
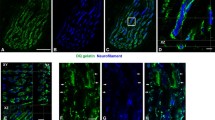
Collagen fiber network is a major contributor to the coherence and tensile strength of normal skeletal muscle. Despite the well-recognized importance of the intramuscular connective tissue to the normal integrity and function of the skeletal muscle, the specific architecture including the location and three-dimensional orientation of the intramuscular connective tissue within the muscle tissue is poorly described. The structure of the intramuscular connective tissue was studied by immunohistochemistry, polarization microscopy (the crimp length and angle of the collagen fibers) and scanning electron microscope (SEM) in rat skeletal muscles (gastrocnemius, soleus and tibialis anterior) in normal situation and after 3 weeks of disuse (immobilization). Three separate networks of collagen fibers were distinguished by SEM in the normal endomysium; fibers running longitudinally on the surface of the muscle fibers (the main collagen orientation), fibers running perpendicularly to the long axis of the muscle fibers and having contacts with adjacent muscle fibers, and fibers attached to the intramuscular nerves and arteries. Similarly, the SEM analysis also disclosed three distinct collagen fiber networks running in different directions in the perimysium, but, contrary to the endomysium, the main fiber orientation could not be established. Immobilization resulted in a marked increase in the endo- and perimysial connective tissue, the majority of the increased endomysial collagen being deposited directly on the sarcolemma of the muscle cells. Immobilization also resulted in substantial increase in the number of perpendicularly oriented collagen fibers with contacts to two adjacent muscle fibers in the endomysium. Further, immobilization clearly disturbed the normal structure of the endomysium making it impossible to distinguish the various networks of fibers from each other. In the perimysium, immobilization-induced changes were similar, the number of longitudinally oriented collagen fibers was increased, the connective tissue was very dense, the number of irregularly oriented collagen fibers was markedly increased, and consequently, the different networks of collagen fibers could not be distinguished from each other. Of the three studied intact muscles, the crimp angle of the collagen fibers was lowest in the soleus and highest in the gastrocnemius muscle, and the crimp angle decreased over 10% in all muscles after the immobilization-period. Altogether, the above described quantitative and qualitative changes in the intramuscular connective tissue are likely to contribute to the deteriorated function and biomechanical properties of the immobilized skeletal muscle.
Similar content being viewed by others
References
Appell H-J (1990) Muscular atrophy following immobilisation. A review. Sports Med 10: 42-58.
Booth FW and Seider MJ (1979) Recovery of skeletal muscle after 3 mo of hindlimb immobilization in rats. J Appl Physiol 47: 435-439.
Booth FW (1978) Regrowth of atrophied skeletal muscle in adult rats after ending immobilization. J Appl Physiol 44: 225-230.
Borisov AB, Huang S and Carlson BM (2000) Remodeling of the vascular bed and progressive loss of capillaries in denervated skeletal muscle. Anat Rec 258: 292-304.
Cohn RD and Campbell KP (2000) Molecular basis of muscular dystrophies. Muscle Nerve 23: 1456-1471.
Desplanches D, Mayet MH, Sempore B, Frutoso J and Flandrois R (1987) Effect of spontaneous recovery of retraining after hindlimb suspension on aerobic capacity. J Appl Physiol 63: 1739-1743.
Diamont J, Keller A, Baer E, Litt M and Arridge RGC (1972) Collagen: ultrasturcture and its relation to mechanical properties as a function of aging. Proc Roy Soc London (Biol) 180: 293-315.
Fitts RH and Brimmer CJ (1985) Recovery in skeletal muscle contractile function after prolonged hindlimb immobilization. J Appl Physiol 59: 916-923.
Frost HM (1990) Skeletal structural adaptations to mechanical usage (SATMU): 4. Mechanical influences on intact fibrous tissues. Anat Rec 226: 433-439.
Goldspink G (1991) Cellular and molecular aspects of adaptation in skeletal muscle. In: Komi P (ed.) Strength and Power in Sports, (vol. 1, pp. 211-229). Blackwell, Oxford.
Han X-Y, Wang W, Myllylä R, Virtanen P, Karpakka J and Takala TES (1999) mRNA levels for α-subunit of prolyl 4-hydroxylase and fibrillar collagens in immobilized rat skeletal muscle. J Appl Physiol 87: 90-96.
Järvinen M (1977) Immobilization effect on the tensile properties of striated muscle: an experimental study in the rat. Arch Phys Med Rehabil 58: 123-127.
Järvinen MJ, Einola SA and Virtanen EO (1992) Effect of the position of immobilization upon the tensile properties of the rat gastrocnemius muscle. Arch Phys Med Rehabil 73: 253-257.
Järvinen TAH, Józsa L, Kannus P, Järvinen TLN, Kvist M, Hurme T, Isola J, Kalimo H and Järvinen M (1999) Mechanical loading regulates tenascin-C expression in the osteotendinous junction. J Cell Sci 112: 3157-3166.
Józsa L, Järvinen M and Réffy A (1980a) A New preparation technique for scanning electron microscopy of skeletal muscle. Microsc Acta 83: 45-47.
Józsa L, Järvinen M, Kvist M, Lehto M and Mikola A (1980b) Capillary density of tenotomized skeletal muscles. I. Experimental study in the rat. Eur J Appl Physiol Occup Physiol 44: 175-181.
Józsa L, Kannus P, Järvinen TAH, Balint J and Järvinen M (1998) Blood flow in rat gastrocnemius muscle and Achilles tendon after Achilles tenotomy. Eur Surg Res 30: 125-129.
Józsa L, Kannus P, Thöring J, Reffy A, Järvinen M and Kvist M (1990) The effect of tenotomy and immobilisation on intramuscular connective tissue. A morphometric and microscopic study in rat calf muscles. J Bone Joint Surg 72B: 293-297.
Józsa L, Thöring J, Järvinen M, Kannus P, Lehto M and Kvist M (1988) Quantitative alterations in intramuscular connective tissue following immobilization: an experimental study in the rat calf muscles. Exp Mol Pathol 49: 267-278.
Kannus P (2000) Structure of the tendon connective tissue. Scand J Med Sci Sports 10: 312-320.
Kannus P, Jozsa L, Kvist M, Lehto M and Järvinen M (1992a) The effect of immobilization on myotendinous junction: an ultrastructural, histochemical and immunohistochemical study. Acta Physiol Scand 144: 387-394.
Kannus P, Jozsa L, Renström P, Järvinen M, Kvist M, Lehto M, Oja P and Vuori I (1992b) The effects of training, immobilization and remobilization on musculoskeletal tissues. 1. Training and immobilization. Scand J Med Sci Sports 2: 100-118.
Kannus P, Jozsa L, Renström P, Järvinen M, Kvist M, Lehto M, Oja P and Vuori I (1992c) The effects of training, immobilization and remobilization on musculoskeletal tissues. 2. Remobilization and prevention of immobilization atrophy. Scand J Med Sci Sports 2: 164-176.
Kannus P, Józsa L, Kvist M, Järvinen T and Järvinen M (1998a) Effects of immobilization and subsequent low-and high-intensity exercise on morphology of rat calf muscles. Scand J Med Sci Sports 8: 160-171.
Kannus P, Józsa L, Järvinen TLN, Kvist M, Vieno T, Järvinen TAH, NatriA and Järvinen M (1998b) Free mobilization and low-to high-intensity exercise in immobilization-induced muscle atrophy. J Appl Physiol 84: 1418-1424.
Kannus P, Parkkari J, Järvinen TLN, Järvinen TAH and Järvinen M (2002) Basic science and clinical studies coincide: active approach is needed in the treatment of sports injuries. (Editorial). Scand J Med Sci Sports (in press).
Karpakka J, Virtanen P, Väänänen K, Orava S and Takala TES (1991) Collagen synthesis in rat skeletal muscle during immobilization and remobilization. J Appl Physiol 70: 1775-1780.
Kasper CE, White TP and Maxwell LC (1990) Running during recovery from hindlimb suspension induces transient muscle injury. J Appl Physiol 68: 533-539.
Kvist M, Hurme T, Kannus P, Järvinen T, Maunu V-M, Jozsa L and Järvinen M (1995) Vascular density at the myotendinous junction of the rat gastrocnemius muscle after immobilization and remobilization. Am J Sports Med 23: 359-364.
O'Brien M (1997) Structure and metabolism of tendons. Scand J Med Sci Sports 7: 55-61.
Tabary TC, Tabary C, Tardieu C, Tardieu G and Goldspink G (1972) Physiological and structural changes in the cat's soleus muscle due to immobilization at different lengths by plaster casts. J Physiol 224: 231-244
Takala TE and Virtanen P (2000) Biochemical composition of muscle extracellular matrix: the effect of loading. Scand J Med Sci Sports 10: 321-325.
Tomanek RJ and Lund DD (1974) Degeneration of different types of skeletal muscle fibres. II. Immobilization. J Anat 118: 531-541.
Tyml K and Mathieu-Costello O (2001) Structural and functional changes in the microvasculature of disused skeletal muscle. Front Biosci 6: D45-D52.
Williams PE and Goldspink G (1978) Changes in sarcomere length and physiological properties in immobilized muscle. J Anat 127: 459-468.
Williams PE and Goldspink G (1981) Connective tissue changes in surgically overloaded muscle. Cell Tissue Res 221: 465-470.
Williams PE and Goldspink G (1984) Connective tissue changes in immobilized muscle. J Anat 138: 343-350.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Järvinen, T.A.H., Józsa, L., Kannus, P. et al. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. J Muscle Res Cell Motil 23, 245–254 (2002). https://doi.org/10.1023/A:1020904518336
Issue Date:
DOI: https://doi.org/10.1023/A:1020904518336