Abstract
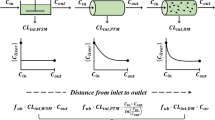
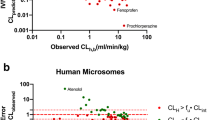
The aim of this investigation is to compare different mathematical models of the liver in the context of in vitro-in vivo correlation. We reanalyze drugs from the Houston reviews [1, 2], and compare the mathematical models. For the well-stirred model, a particular form of the distributed tubes model, and the dispersion model, fits are done to in vitro and in vivo intrinsic clearance data from microsomal and hepatocyte experiments. The distributed and dispersion models have decreased residuals as compared to the well-stirred model, but neither is to be clearly preferred over theother. It seems likely that drug-specific factors have a major impact on the quality of IVIVC correlations. While new experiments are needed to validate IVIVC models, our results indicate that improved correlation of in vitroand in vivo data is possible for high clearance drugs by using either a dispersion or distributed tube model rather than a well-stirred model.
Similar content being viewed by others
References
Houston, J.B.:Utility of In Vitro Drug Metabolism Data in Predicting In Vivo Metabolic Clearance, Biochem. Pharm. 47(9) (1994), 1469-1479.
Houston, J.B. and Carlile, D.J.: Prediction of Hepatic Clearance from Microsomes, Hepatocytes, and Liver Slices, Drug Metab. Rev.29(4) (1997), 891-922.
Gladtke, E. and von Hattingberg, H.M.: Pharmacokinetics: An Introduction,Springer-Verlag, Berlin, 1979.
Schwinghammer, T.L. and Kroboth, P.: Basic Concepts in Pharmacodynamic Modeling, J. Clin. Pharmacol.28 (1988), 388-394.
Colburn, W.A.: Controversy III: To Model or Not to Model, Pharmacokinetics 28 (1988), 879-888.
Winters, M.E.: Basic Clinical Pharmacokinetics, Applied Therapeutics, Inc., Vancouver, WA, 1994.
Gibaldi, M. and Perrier, D.: Pharmacokinetics, Marcel Dekker, 2nd edn, 1988.
Hoang, K.C.T.: Physiologically Based Pharmacokinetic Models: Mathematical Fundamentals and Simulation Implementations, Toxicol. Lett. 79 (1995), 99-106.
Gillespie, W.R.: Noncompartmental versus Compartmental Modelling in Clinical Pharmacokinetics, Clin. Pharmacokin. 20 (1991), 243-262.
Evans, W., Schentag, J. and Jusko, W. (eds.): Applied Pharmacokinetics: Principles of Theraputic Drug Monitoring, Applied Therapeutics, Inc., Vancouver, WA, 3rd edn, 1992.
Gibson, G.G. and Skett, P.: Introduction to Drug Metabolism, Blackie Academic and Professional, an imprint of Chapman & Hall, Glasgow, 1994.
O'Grady, K.F. and Doyle, D.J.: Compartmental Modeling in Clinical Pharmacokinetics, Clin. Enging. 20 (1995), 156-170.
Liang, E. and Derendorf, H.: Pitfalls in Pharmacokinetic Multicompartment Analysis, J. Pharmacokin. Biopharm. 26 (1998), 247-260.
Saville, B.A., Gray, M.R. and Tam, Y.K.: Models of Hepatic Drug Elimination, Drug Metab. Rev. 24(1) (1992), 49-88.
Pang, K.S. and Rowland, M.: Hepatic Clearance of Drugs. I. Theoretical Considerations of a 'Well-Stirred' Model and a 'Parallel Tube' Model. Influence of Hepatic Blood Flow, Plasma and Blood Cell Binding, and the Hepatocellular Enzymatic Activity of Hepatic Drug Clearance, J. Pharmacokin. Biopharm. 5(6) (1977), 625-652.
Roberts, M.S., Donaldson, J.D. and Rowland, M.: Models of Hepatic Elimination: Comparison of Stochastic Models to Describe Residence Time Distributions and to Predict the Influence of Drug Distribution, Enzyme Heterogeneity, and Systemic Recycling on Hepatic Elimination, J. Pharmacokin. Biopharm. 16(1) (1988), 41-83.
Roberts, M.S. and Rowland, M.: A Dispersion Model of Hepatic Elimination: 1. Formulation of the Model and Bolus Considerations, J. Pharmacokin. Biopharm. 14(3) (1986), 227-260.
Hisaka, A. and Sugiyama, Y.: Notes on the Inverse Gaussian Distribution and Choice of Boundary Conditions for the Dispersion Model in the Analysis of Local Pharmacokinetics, J. Pharm. Sci. 88(12) (1999), 1362-1365.
Roberts, M.S., Anissimov, Y.G. and Weiss, M.: Commentary: Using the Convection-Dispersion Model and Transit Time Density Functions in the Analysis of Organ Distribution Kinetics, J. Pharm. Sci. 89(12) (2000), 1579-1586.
Hisaka, A. and Sugiyama, Y.: Problems of Mixed Boundary Conditions for Convection-Dispersion Models in the Analysis of Local Pharmacokinetics, J. Pharm. Sci. 89(12) (2000), 1587-1588.
Weiss, M.: A Note on the Interpretation of Tracer Dispersion in the Liver, J. Theo. Biol. 184 (1997), 1-6.
Anissimov, Y.G., Bracken, A.J. and Roberts, M.S.: Interconnected-tubes Model of Hepatic Elimination, J. Theor. Biol. 188 (1997), 89-101.
Anissimov, Y.G., Bracken, A.J. and Roberts, M.S.: Interconnected-tubes Model of Hepatic Elimination: Steady-State Considerations, J. Theor. Biol. 199 (1999), 435-447.
Bass, L., Roberts, M.S. and Robinson, P.J.: On the Relation Between Extended Forms of the Sinusoidal Perfusion and of the Convection-Dispersion Models of Hepatic Elimination, J. Theor. Biol. 126 (1987), 457-482.
Rane, A., Wilkinson, G.R. and Shand, D.G.: Prediction of Hepatic Extraction Ratio from In Vitro Measurement of Intrinsic Clearance, J. Pharmacol. Exp. Ther. 200(2) (1977), 420-424.
Chiba, M., Fujita, S. and Suzuki, T.: Pharmacokinetic Correlation Between In Vitro Hepatic Microsomal Enzyme Kinetics and In Vivo Metabolism of Imipramine and Desipramine in Rats, J. Pharma. Sci. 79(4) (1990), 281-287.
Kukan, M., Bezek, S., Pool, W.F. and Woolf, T.F.: Metabolic Disposition of Tacrine in Primary Suspensions of Rat Hepatocyte and in Single-Pass Perfused Liver: In Vitro/In Vivo Comparisons, Xenobiotica 24(11) (1994), 1107-1117.
Sawada, Y., Hanano, M., Sugiyama, Y. and Iga, T.: Prediction of the Disposition of NineWeakly Acidic Drugs and Six Weekly Basic Drugs in Humans from Pharmacokinetic Parameters in Rats, J. Pharmacokin. Biopharm. 13(5) (1985), 477-492.
Worboys, P.D., Brennan, B., Bradbury, A. and Houston, J.B.:Metabolite Kinetics of Ondansetron in Rat. Comparison of Hepatic Microsomes, Isolated Hepatocytes, with In Vivo Disposition, Xenobiotica 26(9) (1996), 897-907.
Carlile, D.J., Stevens, A.J., Ashforth, E.I.L., Waghela, D. and Houston, J.B.: In Vivo Clearance of Ethoxycoumarin and Its Prediction from In Vitro Systems: Use of Drug Depletion and Metabolite Formation Methods in Hepatic Microsomes and Isolated Hepatocytes, Drug Metab. Dispos. 26(3) (1998): 216-221.
Belpaire, F.M., Smet, F.D., Chindavijak, B., Fraeyman, N. and Bogaert, M.G.: Effect of Turpentine-Induced Inflammation on the Disposition Kinetics of Propranolol, Metoprolol, and Antipyrine in the Rat, Fundam. Clin. Pharmacol. 3 (1989), 79-88.
Zomorodi, K., Carlile, D.J. and Houston, J.B.: Kinetics of Diazepam Metabolism in Rat Hepatic Microsomes and Hepatocytes and Their Use in Predicting In Vivo Hepatic Clearance, Xenobiotica 25(9) (1995), 907-916.
Gelman, C.R., Rumack, B.H. and Hess, A.J. (eds.): DRUGDEX System ®. MICROMEDEX Inc., Englewood, Colorado, 1999.
Obach, R.S.: Nonspecific Binding to Microsomes: Impact on Scale-up of In Vitro Intrinsic Clearance to Hepatic Clearance as Assessed Through Examination of Warfarin, Imipramine, and Propranolol, Drug Met. Disp. 25(12) (1997), 1359-1369.
Obach, R.S., Baxter, J.G., Liston, T.E., Silber, B.M., Jones, B.C., MacIntyre, F., Rance, D.J. and Wastall, P.: The Prediction of Human Pharmacokinetic Parameters from Preclinical and In Vitro Metabolism Data, J. Pharmac. Exp. Thr. 283(1) (1997), 46-58.
Bass, L.: Saturation Kinetics in Hepatic Drug Removal: A Statistical Approach to Functional Heterogeneity, Am. J. Physiol. 244 (1983), 6583-6589.
Morgan, D.J. and Raymond, K.: Use of Unbound Drug Concentration in Blood to Discriminate Between Two Models of Hepatic Drug Elimination, J. Pharm. Sci. 71(5) (1982), 600-602.
Roberts, M.S. and Rowland, M.: A Dispersion Model of Hepatic Elimination: 2. Steady-State Considerations - Influence of Hepatic Blood Flow, Binding Within Blood, and Hepatocellular Enzyme Activity, J. Pharmacokin. Biopharm. 14(3) (1986), 261-288.
Roberts, M.S. and Rowland, M.: Correlation Between In-Vitro Microsomal Enzyme Activity and Whole Organ Hepatic Elimination Kinetics: Analysis with a Dispersion Model, J. Pharm. Pharmacol. 38(3) (1986), 177-181.
Ridgway, D., Tuszynski, J. and Tam, Y.K.: Modeling In Vivo-In Vitro Correlation, PharmSci Supp. 1(1) (1998), S-488. Abstract presented at 1998 AAPS Annual Meeting.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ridgway, D., Tuszynski, J. & Tam, Y. Reassessing Models of Hepatic Extraction. Journal of Biological Physics 29, 1–21 (2003). https://doi.org/10.1023/A:1022531403741
Issue Date:
DOI: https://doi.org/10.1023/A:1022531403741