Abstract
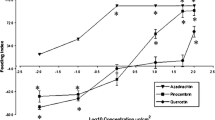
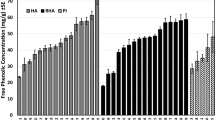
A methanol extract of the pod surfaces of Cajanus cajan, a feeding stimulant for fifth-instar Helicoverpa armigera, was shown to contain four main phenolic compounds. Three of these were identified as isoquercitrin, quercetin, and quercetin-3-methyl ether, by comparing UV spectra and HPLC retention times with authentic standards. The fourth compound was isolated by semipreparative HPLC and determined to be 3-hydroxy-4-prenyl-5- methoxystilbene-2-carboxylic acid (stilbene) by NMR spectroscopy and mass spectrometry. Quercetin, isoquercitrin, and quercetin-3-methyl did not affect the selection-behavior of fifth-instar H. armigera. However, larvae were deterred from feeding on glass-fiber disks impregnated with the stilbene. Furthermore, larvae exposed to quercetin-3-methyl ether consumed significant amounts of both disks. In a binary-choice bioassay, a combination of quercetin-3-methyl ether and the stilbene on one disk and pure quercetin-3-methyl ether on the other disk resulted in increased consumption of both glass-fiber disks by larvae. In contrast, consumption was reduced if the combination was presented to larvae on one disk with purified stilbene on the other disk. Cajanus cajan cultivars that varied in their susceptibility to H. armigera were surveyed for the presence of the four phenolic compounds. An absence of quercetin and higher concentrations of isoquercitrin than the cultivated variety characterized pod surface extracts of pod-borer-resistant cultivars. In addition, the ratio of the stilbene to quercetin-3-methyl ether was greater in the pod-borer-resistant cultivars. These findings are discussed in relation to the identification of chemical characters that can be used for crop improvement.
Similar content being viewed by others
References
Armes, N. J., Bond, G. S., and Cooter, R. J. 1992. The laboratory culture and development of Helicoverpa armigera. Natural Resources Institute Bulletin No. 57. Natural Resources Institute, Chatham, United Kingdom.
Blaney, W. M. and Simmonds, M. S. J. 1983. Electrophysiological activity in insects in response to antifeedants. COPR Project no. 9, Final Report. ODA, United Kingdom.
Carter, M., Sachdev-Gupta, K., and Feeny, P. 1998. Tyramine from the leaves of wild parsnip: a stimulant and a synergist for oviposition by the black swallowtail butterfly. Physiol. Entomol. 23:303–312.
Cooksey, C. J., Dahiya, J. S., Garratt, P. J., and Strange, R. N. 1982. Two novel stilbene-2-carboxylic acids from Cajanus cajan. Phytochemistry 21:2935–2938.
Faini, F., Labbe, J., Salgado, I., and Coll, J. 1997. Chemistry, toxicity and antifeedant activity of the resin of Flourensia thurifera. Biochem. Syst. Ecol. 25:189–193.
Feeny, P., Sachdev, K., Rosenberry, L., and Carter, M. 1988. Luteolin-7-O-(6′-O-malonyl)-β-d-glucoside and trans-chlorogenic acid: oviposition stimulants for the black swallowtail butterfly. Phytochemistry 27:3439–3448.
Gorham, J. 1989. Stilbenes and Phenathrenes, pp. 159–196, in P. M. Dey and J. B. Harborne (Eds.). Methods in Plant Biochemistry, Volume 1: Plant Phenolics. Academic Press, London, United Kingdom.
Grayer, R. J., Kimmins, F. M., Stevenson, P. C., Harborne, J. B., and Wijayagunesekera, H. N. P. 1994. Phenolics in rice phloem sap as sucking deterrents to the brown planthopper (Nilaparvata lugens). Acta Hortic 381:391–394.
Green, P. W. C., Stevenson, P. C., Simmonds, M. S. J., and Sharma, H. C. 2002. Can larvae of the pod-borer, Helicoverpa armigera (Lepidoptera: Noctuidae), select between wild and cultivated pigeonpea [Cajanus sp. (Fabaceae)]? Bull. Entomol. Res. 92:45–51.
Harborne, J. B., Baxter, H, and Moss, G. P. 1999. Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plants. Taylor and Francis, London, United Kingdom.
Hamamura, Y., Matsuura, K., Nishida, J., Hayashiya, K., and Naito, K. I. 1962. Food selection by silkworm larvae. Nature 194:754.
Lateef, S. S. and Reed, W. 1990. Insect pests of pigeonpea, pp. 193–242, in S. R. Singh (ed.). Insect Pests of Tropical Food Legumes. John Wiley & Sons, New York.
Lindroth, R. L. and Peterson, S. S. 1988. Effects of plant phenols on southern armyworm larvae. Oecologia 755:185–189.
Ranga Rao, G. V. and Shanower, T. G. 1999. Identification and management of pigeonpea and chickpea insect pests in Asia. ICRISAT Information Bulletin no. 57. International Crop Research Institute for the Semi-Arid Tropics, Andhra Pradesh, India.
Roessingh, P., Stadler, E., Schoni, E., and Feeny, P. 1991. Tarsal contact chemoreceptors of the black swallowtail butterfly, Papilio polyxenes: responses to phytochemicals from host-and non-host plants. Physiol. Entomol. 16:485–495.
Shanower, T. G., Yoshida, M., and Peter, A. J. 1997. Survival, fecundity and behavior of Helicoverpa armigera (Lepidoptera: Noctuidae) on pigeonpea and two wild Cajanus species. J. Econ. Entomol. 90:837–841.
Shanower, T. G., Romeis, J., and Minja, E. M. 1999. Insect pests of pigeonpea and their management. Annu. Rev. Entomol. 44:77–96.
Sharma, H. C., Green, P. W. C., Stevenson, P. C., and Simmonds, M. S. J. 2001. What makes it tasty for the pest? Identification of Helicoverpa armigera (Hübner) feeding stimulants and location of their production on the pod-surface of pigeonpea [Cajanus cajan (L.) Millsp.]. Competitive Research Facility Project R7029 C, Final Technical Report. DFID, London, United Kingdom.
Simmonds, M. S. J. and Stevenson, P. C. 2001. Effects of isoflavonoids from Cicer on larvae of Helicoverpa. J. Chem. Ecol. 27:965–977.
Simmonds, M. S. J., Blaney, W. M., and Fellows, L. E. 1990. Behavioral and electrophysiological study of antifeedant mechanisms associated with polyhydroxyalkaloids. J. Chem. Ecol. 16:3167–3196.
Sokal, R. R. and Rohlf, F. J. 1987. Biostatistics, 2nd ed. W. H. Freeman and Co., New York.
Stevenson, P. C. 1993. Biochemical resistance in wild species of groundnut to Spodoptera litura (Fabr.). Bull. OILB/SROP 16:155–162.
Stevenson, P. C., Kimmins, F. M., Grayer, R. J., and Raveendranath, S. 1996. Schaftosides from rice phloem as feeding inhibitors and resistance factors to brown planthoppers (Nilaparvata lugens). Entomol. Exp. Appl. 80:246–249.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Green, P.W.C., Stevenson, P.C., Simmonds, M.S.J. et al. Phenolic Compounds on the Pod-Surface of Pigeonpea, Cajanus cajan, Mediate Feeding Behavior of Helicoverpa armigera Larvae. J Chem Ecol 29, 811–821 (2003). https://doi.org/10.1023/A:1022971430463
Issue Date:
DOI: https://doi.org/10.1023/A:1022971430463