Abstract
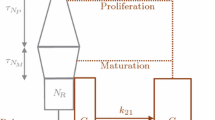
Basic indirect pharmacodynamic models for agents which alter the generation of natural cells based on a life-span concept are introduced. It is assumed that cells (R) are produced at a constant rate (k in ), survive for a specific duration T R , and then are lost. The rate of cell loss must equal the production rate but is delayed by T R , A therapeutic agent can stimulate or inhibit the production rate according to the Hill function: 1 ±H(C(t)) where H(C(t)) contains capacity (S max ) and sensitivity (SC 50 ) constants and C(t) is a pharmacokinetic function. Thus an operative model is dR/dt=k in · [1± H(C(t))]-kin ·[1 ± H(C(t-TR))] with the baseline condition R0 = k in · T R . One- and two-compartment catenary cell models were examined by simulation to describe the role of pharmacokinetics and cell properties. The area under the effect curve (AUCE) was derived. The models were applied to literature data to describe the stimulatory effects of single doses of hematopoietic growth factors such as granulocyte colony-stimulating factor (G-CSF) on neutrophils, thrombopoietin (TPO) on platelets, and erythropoietin (EPO) on reticulocytes in blood. The models described experimental data adequately and provided cell life-spans and SC50 values. The proposed cell production/loss models can be readily used to analyze the pharmacodynamics of agents which alter cell production yielding realistic physiological parameters.
Similar content being viewed by others
REFERENCES
H. E. Wichmann and M. Loeffler. Mathematical Modeling of Cell Proliferation: Stem Cell Regulation in Hemopoiesis, Vol. 1, CRC Press, Boca Raton, FL, 1985.
M. Loeffler, K. Pantel, H. Wulff, and H. E. Wichmann. A mathematical model of erythropoiesis in mice and rats, Part 1: Structure of the model. Cell Tissue Kinet. 22:13-30 (1989).
H. E. Wichmann, M. Loeffler, K. Pantel, and H. Wulff. A mathematical model of erythropoiesis in mice and rats, Part 2: Stimulated erythropoiesis. Cell Tissue Kinet. 22:31-49 (1989).
H. Wulff, H. E. Wichmann, K. Pantel, and M. Loeffler. A mathematical model of erythropoiesis in mice and rats, Part 3: Suppressed erythropoiesis. Cell Tissue Kinet. 22:51-61 (1989).
K. Pantel, and M. Loeffler, B. Bungart, and H. E. Wichmann. A mathematical model of erythropoiesis in mice and rats, Part 4: Differences between bone marrow and spleen. Cell Tissue Kinet. 23:283-297 (1990).
S. Schmitz, H. Franke, J. Brusis, and H. E. Wichmann. Quantification of the cell kinetic effects of G-CSF using a model of human granulopoiesis. Exp. Hematol. 21:755-760 (1993).
H. E. Wichmann, M. D. Gerhardts, H. Spechtmeyer, and R. Gross. Mathematical model of thrombopoiesis in rats. Cell Tissue Kinet. 12:551-567 (1979).
L. B. Sheiner, D. R. Stanski, S. Vozeh, R. D. Miller, and J. Ham. Simultaneous modeling of pharmacokinetics and pharmacodynamics: Application to d-tubocurarine. Clin. Pharmacol. Ther. 25:358-371 (1979).
N. L. Dayneka, V. Garg, and W. J. Jusko. Comparison of four basic models of indirect pharmacodynamic responses. J. Pharmacokin. Biopharm. 21:457-478 (1993).
F. Bressolle, M. Audran, R. Gareau, T. N. Pham, and R. Gomeni. Comparison of direct and indirect population pharmacodynamic model: Application to recombinant human erythropoietin in athletes. J. Pharmacokin. Biopharm. 25:263-275 (1997).
D. Z. D'Argenio and A. Shumitzky. ADAPT II User's Guide, Biomedical Simulations Resource, University of Southern California, Los Angeles, 1988.
G. D. Demetri and J. D. Griffin. Granulocyte colony-stimulating factor and its receptor. Blood 78:2791 (1991).
N. Stute, V. M. Santana, J. H. Rodman, M. J. Schell, N. J. Ihle, and W. E. Evans. Pharmacokinetics of subcutaneous recombinant human granulocyte colony-stimulating factor. Blood 79:2849-2854 (1992).
T. Kuwabara, S. Kobayashi, and Y. Sugiyama. Pharmacokinetics and pharmacodynamics of a recombinant human granulocyte colony-stimulating factor. Drug Metab. Rev. 28:625-658 (1996).
N. Hayashi, H. Kinoshita, E. Yukawa, and S. Higuchi. Pharmacokinetic and pharmacodynamic analysis of subcutaneous recombinant human granulocyte colony stimulating factor (lenograstim) administration. J. Clin. Pharmacol. 39:583-592 (1999).
N. Kubota, T. Orita, K. Hattori, M. Oh-eda, N. Ochi, and T. Yamazaki. Structural characterization of natural and recombinant human granulocyte colony-stimulating factors. J. Biochem. 107:486-492 (1990).
G. S. Chatta, T. H. Price, R. C. Allen, and D. C. Dale. Effects of in vivo recombinant methionyl human granulocyte colony-stimulating factor on the neutrophil response and peripheral blood colony-forming cells in healthy young and elderly adult volunteers. Blood 84:2923-2929 (1994).
L. K. Roskos, E. N. Cheung, M. Vincent, M. A. Foote, and G. Morstyn. Pharmacology of filgrastim (r-metHuG-CSF), In G. Morstyn, T. M. Dexter, and M. A. Foote (eds.),-Filgrastim (r-metHug-CSF) in Clinical Practice, Marcel Dekker, New York, 1998.
K. Kaushansky. Thrombopoietin: The primary regulator of platelet production. Blood 86:419-431 (1995).
M. Chang, Y. Suen, G. Meng, J. S. Buzby, J. Bussel, V. Shen, C. van de Ven, and M. S. Cairo. Differential mechanisms in the regulations of endogenous levels of thrombopoietin and interleukin-11 during thrombocytopenia; insights into the regulation of platelet production. Blood 89:2782-2788 (1997).
P. J. Fielder, A. L. Gurney, E. Stefaniach, M. Marian, M. W. Moore, K. Carver-Moore, and F. J. de Sauvage. Regulation of thrombopoietin levels by c-mpl-mediated binding to platelets. Blood 87:2154-2161 (1996).
S. Vadhan-Raj, L. J. Murray, C. Bueso-Ramos, S. Patel, S. P. Reddy, W. K. Hoots, T. Johnston, N. E. Papadopolous, W. N. Hittelman, D. A. Johnston, T. A. Yang, V. E. Paton, R. L. Cohen, S. D. Hellmann, R. S. Benjamin, and H. E. Broxmeyer. Stimulation of megakaryocyte and platelet production by a single dose of recombinant human thrombopoietin in patients with cancer. Ann. Intern. Med. 126:673-681 (1997).
W. K. Cheung, B. L. Goon, M. C. Guilfoyle, and M. C. Wacholtz. Pharmacokinetics and pharmacodynamics of recombinant human erythropoietin after single and multiple subcutaneous doses to healthy subjects. Clin. Pharmacol. Ther. 64:412-423 (1998).
R. Berkow (ed.). The Merck Manual of Diagnosis and Therapy, 14th ed., Merck Sharp & Dohme Research Laboratories, Rahway, NJ, 1982.
A. Sharma and W. J. Jusko. Characterization of four basic models of indirect pharmacodynamic responses. J. Pharmacokin. Biopharm. 24:611-634 (1996).
E. A. Murphy and M. E. Francis. The estimation of blood platelets survival: I. General principles of the study of cell survival. Thrombos. Diathes. Haemorrh. 22:281-295 (1969).
W. Krzyzanski and W. J. Jusko. Mathematical formalism for the properties of four basic models of indirect pharmacodynamic responses. J. Pharmacokin. Biopharm. 25:107-123 (1997).
J. V. S. Gobburu and W. J. Jusko. Role of dosage regimen in controlling indirect pharmacodynamic responses, Adv. Drug Delivery Rev. 33:221-233 (1998).
W. Krzyzanski and W. J. Jusko. Integrated functions for four basic models of indirect pharmacodynamic response. J. Pharm. Sci. 87:67-72 (1998).
W. Krzyzanski and W. J. Jusko. Application of moment analysis to the sigmoid effect model for drug administered intravenously. Pharm. Res. 14:949-952 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krzyzanski, W., Ramakrishnan, R. & Jusko, W.J. Basic Pharmacodynamic Models for Agents That Alter Production of Natural Cells. J Pharmacokinet Pharmacodyn 27, 467–489 (1999). https://doi.org/10.1023/A:1023249813106
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1023249813106