Abstract
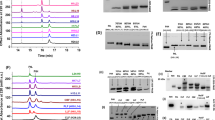
Ferritin utilizes ferroxidase activity to incorporate iron. Iron uptake kinetics of bovine spleen apoferritin were compared with those of recombinant H chain ferritin and L chain ferritin homopolymers. H chain ferritin homopolymer showed an iron uptake rate identical to bovine spleen apoferritin (0.19 and 0.21 mmol/min/μmol of protein, respectively), and both showed iron concentration-dependent uptake. In contrast, the L chain homopolymer, which lacks ferroxidase, did not incorporate iron and showed the same level of iron autoxidation in the absence of ferritin. Bovine spleen apoferritin was shown to have two iron concentration-dependent uptake pathways over a range of 0.02–0.25 mM ferrous ammonium sulfate (FAS) by an Eadie-Scatchard plot (v/[FAS] versus v), whereas the H chain ferritin homopolymer was found to have only one pathway. Of the two Km values found in bovine spleen apoferritin, the lower mean Km value was 9.0 μM, while that of the H chain homopolymer was 11.0 μM. H chain ferritin homopolymer reached a saturating iron uptake rate at 0.1 mM FAS, while bovine spleen apoferritin incorporated more iron even at 0.25 mM FAS. These results suggest that the intrinsic ferroxidase of ferritin plays a significant role in iron uptake, and the L chain cooperates with the H chain to increase iron uptake.
Similar content being viewed by others
References
Andrews SC, Arosio P, Bottke W, Briat JF, von Darl M, Harrison PM, LaulhÈre JP, Levi S, Lobreaux S, Yewdall SJ. 1992 Structure, function and evolution of ferritins. J Inorg Biochem 47, 161-174.
Arosio P, Adelman TG, Drysdale JW. 1978 On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem 253, 4451-4458.
Cairo G, Tacchini L, Pogliaghi G, Anzon E, Tomasi A, Bernelli-Zazzera A. 1995 Induction of ferritin synthesis by oxidative stress. Transcriptional and post-transcriptional regulation by expansion of the “free” iron pool. J Biol Chem 270, 700-703.
Epsztejn S, Kakhlon O, Glickstein H, Breuer W, Cabantchik ZI. 1997 Fluorescence analysis of the labile iron pool of mammalian cells. Anal Biochem 248, 31-40.
Ferreira C, Bucchini D, Martin ME, Levi S, Arosio P, Grandchamp B, Beaumont C. 2000 Early embryonic lethality of H ferritin gene deletion in mice. J Biol Chem 275, 3021-3024.
Harrison PM, Arosio P. 1996 The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1275, 161-203.
Kakuta K, Orino K, Yamamoto S, Watanabe K. 1997 High levels of ferritin and its iron in fetal bovine serum. Comp Biochem Physiol 118A, 165-169.
Laemmli UK. 1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685.
Levi S, Luzzago A, Cesareni G, Cozzi A, Franceschinelli F, Albertini A, Arosio P. 1988 Mechanism of ferritin iron uptake: activity of the H-chain and deletion mapping of the ferro-oxidase site. A study of iron uptake and ferro-oxidase activity of human liver, recombinant H-chain ferritins, and of two H-chain deletion mutants. J Biol Chem 263, 18086-18092.
Levi S, Salfeld J, Franceshinelli F, Cozzi A, Dorner MH, Arosio P. 1989 Expression and structural and functional properties of human ferritin L-chain from Escherichia coli. Biochemistry 28, 5179-5184.
Levi S, Santambrogio P, Cozzi A, Rovida E, Corsi B, Tamborini E, Spada S, Albertini A, Arosio P. 1994 The role of the L-chain in ferritin iron incorporation studies of homo and heteropolymers. J Mol Biol 238, 649-654.
Levi S, Yewdall SJ, Harrison PM, Santambrogio P, Cozzi A, Rovida E, Albertini A, Arosio P. 1992 Evidence that H-and L-chains have co-operative roles in the iron-uptake mechanism of human ferritin. Biochem J 288, 591-596.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951 Protein measurement with the Folin reagent. J Biol Chem 193, 265-275.
Macara IG, Hoy TG, Harrison, PM. 1973 The formation of ferritin from apoferritin. Catalytic action of apoferritin. Biochem J 135, 343-348.
McCord JM. 1996 Effects of positive iron status at a cellular level. Nutrition Rev 54, 85-88.
Orino K, Eguchi K, Nakayama T, Yamamoto S, Watanabe K. 1997 Sequencing of cDNA clones that encode bovine ferritin H and L chains. Comp Biochem Physiol 118B, 667-673.
Orino K, Kamura S, Natsuhori M, Yamamoto S, Watanabe K. 2002a Two pathways of iron uptake in bovine spleen apoferritin dependent on iron concentration. BioMetals 15, 59-63.
Orino K, Higa M, Fujikawa M, Takehara K, Okano S, Yamamoto S, Watanabe K. 2002b Characterization of autoantibodies to ferritin in canine serum. BioMetals 15, 161-165.
Orino K, Tsuji Y, Torti FM, Torti SV. 1999 Adenovirus E1A blocks oxidant-dependent ferritin induction and sensitizes cells to prooxidant cytotoxicity. FEBS Lett 461, 334-338.
Rucker P, Torti FM, Torti SV. 1996 Role of H and L subunits in mouse ferritin. J Biol Chem 271, 33352-33357.
Sun S, Chasteen ND. 1992 Ferroxidase kinetics of horse spleen apoferritin. J Biol Chem 267, 25160-25166.
Theil EC. 1987 Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem 56, 289-315.
Yamaoka K, Tanigawara Y, Nakagawa T, Uno T. 1981 A pharmacokinetic analysis program (MULTI) for microcomputer. J Pharmacobio-Dyn 4, 875-885.
Yang X, Chen-Barrett Y, Arosio P, Chasteen ND. 1998 Reaction paths of iron oxidation and hydrolysis in horse spleen and recombinant human ferritins. Biochemistry 37, 9743-9750.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orino, K., Harada, S., Natsuhori, M. et al. Kinetic analysis of bovine spleen apoferritin and recombinant H and L chain homopolymers: Iron uptake suggests early stage H chain ferroxidase activity and second stage L chain cooperation. Biometals 17, 129–134 (2004). https://doi.org/10.1023/B:BIOM.0000018379.20027.78
Issue Date:
DOI: https://doi.org/10.1023/B:BIOM.0000018379.20027.78