Abstract
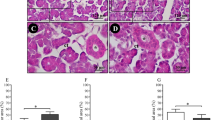
Xerostomia (oral dryness sensation) is due to dryness of the oral cavity and it is more prevalent in the elderly. This study investigated the effect of ageing on parotid gland structure and function of control (2–6 months) and aged (12, 16–18 and 22–24 months) rats employing light microscopic, colorimetric, gas chromatographic and microspectrofluorimetric methods to investigate the morphological changes of the parotid glands, amylase release, endogenous lipid distribution and cytosolic free calcium levels, respectively. When compared to controls, age-related changes were apparent in glands obtained from rats aged 16–18 and 22–24 months, which included reduced acinar cell distribution, enlarged parotid ducts with fatty and connective tissue and mast cell infiltrations. Parotid acini from 12, 16–18 and 22–24-month-old glands showed significant (p < 0.05) age-related decreases in amylase release, compared to controls when challenged with acetylcholine (ACh). No change in basal calcium signals was observed in parotid acini from 2–6 to 16–18-month-old-animals. However, stimulation of 16–18-month-old parotid acini with 10−5M ACh resulted in a significant (p < 0.001) decrease in both peak and plateau phases of the cytosolic Ca2+ signal when compared to control. Gas chromatography of de novo and essential acyl lipids revealed no changes in the amount of either acyl lipid group in glands obtained from 2–6 to 22–24-month-old animals. Lipid analysis of phospholipid associated acyl chains showed a higher relative proportion of linoleic acid in older glands. The results reveal that ageing is associated with marked and distinct morphological changes including infiltrations of lipids and mast cells of the parotid gland and decreases in amylase release and cytosolic Ca2+ signals (Mol Cell Biochem 266: 199–208, 2004)
Similar content being viewed by others
References
Draper E, Singh J, Brown J, Pallot DJ, Parvez H, Adeghate EA: Morphological and physiological investigation of the effect of age on the rat lacrimal gland. Biogenic Amines 13: 509–523, 1997
Draper CE, Adeghate E, Singh J, Pallot DJ: Evidence to suggest morphological and physiological alteration of lacrimal gland with ageing. Exp Eye Res 68: 265–276, 1999
Draper CE, Singh J, Lawrence NA, Garner A, Pallot DA, Adeghate E: Agerelated morphological changes and secretory responses in male rat lacrimal gland. J Auton Nerv Syst 69: 173–183, 1998
Rattan SIS: Ageing-a biological perspective. Mol Aspect Med 16: 439–508, 1995
Wolf A, Fox PC, Ship JA, Atkinson JC, Macynski RN, Baum BJ: Oralmucosal status and major salivary gland function. Oral Surg Oral Med Oral Pathol 70: 49–54, 1990
Meisel LM, Skobe Z, Prostak PS, Shklar G: A light and electron microscope study of aging parotid and submandibular salivary glands of Swiss-Webster mice. Exp Gerontol 23: 197–210, 1988
Niedermeier W, Huber M, Fischer D, Beier K, Muller N, Schuler R, Brinninger A, Fartasch M, Diegen T, Matthaeus C, Hecto MP: Significance of saliva for the denture-wearing population. Gerontology 17: 104–118, 2000
Caplan DJ, Hunt RJ: Salivary flow and risk of tooth loss in an elderly population. Comm Dent Oral Epidemiol 24: 68–71, 1996
Scully C: Sjogren's syndrome: Clinical and laboratory features, immunopathogenesis and management. Oral Surg Oral Med Oral Pathol 62: 510–523, 1986
Petersen OH: Stimulus-secretion coupling: Cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J Physiol 448: 1–51, 1992
Schultz I: Stimulus-secretion coupling in exocrine glands: Role of in 1,4,5-trisphosphate, calcium and camp. Curr Eye Res 4: 467–473, 1985
Bodner L, Hoops MT, Gee M, Ito H, Roth GS, Baum BJ: Multiple trans-duction mechanisms are likely involved in calcium-mediated exocrine secretory events in rat parotid cells. J Biol Chem 258: 2774–2777, 1983.
Puntey Jr JW: Identification of cellular activation mechanisms associated with salivary secretion. Ann Rev Physiol 4: 75–88, 1986
Ambudkar IS: Regulation of calcium in salivary gland secretion. Crit Rev Oral Biol Med 11: 4–25, 2000
Kameyama Y, Yashiro K, Mizuno M, Okada A, Takahashi K, Yokota Y:Comparison of membrane phospholipid and its fatty acid compositions in developing rat salivary glands. Comp Biochem Physiol Biol 87: 741–746, 1987
Adeghate E, Singh J, Burrows S, Howarth FC, Donath T: Secretory responses and peptidergic and aminergic innervation of the rat lacrimal glands. Biogenic Amines 10: 487–498, 1994
Karnovsky MJ: A formaldehyde-glutaraldehyde fixative of light osmolality for use in electron microscopy. J Cell Biol 27: 137A, 1965
Gonzalez A, Camello PJ, Pariente JA, Salido GM: Free cytosolic calcium levels modify intracellular pH in rat acini. Biochem Biophys Res Commun 230: 652–656, 1997
Herzog V, Sies H, Miller F: Exocytosis in secretory cells of rat lacrimalgland. J Cell Biol 70: 692–706, 1976
Gardner JD, Jackson MJ: Regulation of amylase release from dispersedpancreatic acinar cells. J Physiol 270: 439–454, 1977
Ceska M, Birath K, Brown B: A new and rapid method for the clinical determination of β-amylase activities in human serum and urine. Optimal conditions. Clin Chem Acta 26: 437–444, 1969
Pariente JA, Camello C, Camello PJ, Salido GM: Release of calcium from mitochondrial and non-mitochondrial intracellular stores in mouse pancreatic acinar cells by hydrogen peroxide. J Membr Biol 179: 27–35, 2000
Rolph EC, Goad LJ: Phosphatidylcholine biosynthsesis in celery cell suspension cultures with altered sterol composistions. Physiol Plant 83: 605–610, 1991
Kim SE: Age-related changes in cellular level of amylase and protein synthesis in the rat parotid gland. J Dent Res 60: 738–747, 1981
Moron G, Maletto B, Orsilles M, Depiante-Depaoli M, Pistoresi-Palencia MC: Age-related alterations in inflammatory response during experimental autoimmune prostatitis. Mech Ageing Dev 118: 71–85, 2000
Mizuhira V, Hasegawa H, Notoya M: Fixation and imaging of biological elements: Heavy metals, diffusible substances, ions, peptides, and lipids. Prog Histochem Cytochem 35: 67–183, 2000
Baum BJ: Neurotransmitter control of secretion. J Dent Res 66: 628–632, 1987
Chen C, Noland KA, Kalu DN: Effects of food restriction on alpha 1-adrenergic receptors and G-protein in the rat parotid gland. J Gerontol Biol Sci Med Sci 52: B103–B110, 1997
Ishikawa Y, Gee MV, Ambudkar IS, Bodner L, Baum BJ, Roth GS: Age-related impairment in rat parotid cell β 1-adrenergic action at the level on inositol trisphosphate responsiveness. Biochem Biophys Acta 968: 203–210, 1988
Sawaki K, Baum BJ, Ambudkar IS: Decreased m3-muscarinic and alpha 1-aderenergic receptor stimulation of PIP2 hydrolysis in parotid gland membranes from aged rats: Defect in activation of G alpha q/11. Arch Biochem Biophys 332: 319–326, 1995
Baum BJ, Levine RL, Kuyatt, BL, Sogin DB: Rat parotid gland amylase: Evidence for alteration in an exocrine protein with increased age. Mech Ageing Dev 19: 27–35, 1982
Baum BJ, Bodner L: Characteristics of stimulated parotid gland secretionin the ageing rat. Mech Ageing Dev 31: 337–342, 1985
Koller MM, Maeda N, Purushotham KR, Scarpace PJ, Humphreys-Beher MG: A biochemical analysis of parotid and submandibular salivary gland function with age after simultaneous stimulation with pilocarpine and isoproterenaol in female NIA Fischer 344 rats. Arch Oral Biol 37: 219–230, 1992
Salih MA, Kalu DN, Smith TC: Effects of age and food restriction on calcium signalling in parotid acinar cells of Fischer 344 rats. Ageing (Milano) 9: 419–427, 1997
Maki T, Kowatch MA, Baum BJ, Ambudkar IS, Roth GS: Evidence for an alteration in the microsomal Ca2+ release mechanism in senes-cent rat parotid acianr cells. Biochem Biophys Acta 1014: 73–77, 1989
Murawski U, Kriesten K, Egge H: Age-related changes of lipid fractions and total fatty acids in liver lipids and heart lipids of female and male rats aged 37–1200 days (liver) and 331–1200 days (heart). Comp Biochem Physiol Biol 96: 271–289, 1990
Kates AM, Herrero P, Dence C, Soto P, Srinivasan M, Delano DG, Ehsani A, Gropler RJ: Impact of aging on substrate metabolism by the human heart. J Am Coll Cardiol 41: 293–299, 2003
Montine TJ, Neely MD, Quinn JF, Beal MF, Markesbery WR, Roberts LJ, Morrow JD: Lipid peroxidation in aging brain and Alzheimer's disease. Free Radic Biol Med 33: 620–626, 2002
Chvojkova S, Kazdova L, Divisova J: Age-related changes in fatty acid composition in muscles. Tohoku J Exp Med 195: 115–123, 2001
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mahay, S., Pariente, J.A., Lajas, A.I. et al. Effects of ageing on morphology, amylase release, cytosolic Ca2+ signals and acyl lipids in isolated rat parotid gland tissue. Mol Cell Biochem 266, 199–208 (2004). https://doi.org/10.1023/B:MCBI.0000049158.85447.4f
Issue Date:
DOI: https://doi.org/10.1023/B:MCBI.0000049158.85447.4f