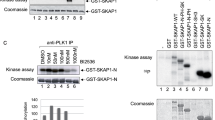
Abstract
Signaling lymphocyte activation molecule (SLAM)-associated protein (SAP) is a short intracellular molecule that is mutated in humans with X-linked lymphoproliferative (XLP) disease. Although the exact role and mechanism of action of SAP are not known, it has the capacity to interact with the cytoplasmic region of SLAM and other related immune cell receptors. As SAP is composed almost exclusively of a Src homology 2 (SH2) domain, it has been proposed that it functions as a natural blocker of SH2 domain–mediated interactions. We report here that the SLAM receptor is capable of triggering a protein tyrosine phosphorylation signal in T cells via a mechanism that is strictly dependent on SAP expression. This signal involves the SH2 domain–containing inositol phosphatase (SHIP); the adaptor molecules Dok2, Dok1 and Shc; and Ras GTPase–activating protein RasGAP. SAP is essential for this pathway because it facilitates the selective recruitment and activation of the Src-related protein tyrosine kinase FynT. We also show that signaling via the SLAM-SAP pathway in an established T cell line can alter the profile of cytokine production during T cell activation. These findings identify a mechanism by which a putative adaptor molecule is required for receptor-mediated signaling events in the immune system. They also provide insights into the pathophysiology of a severe human lymphoproliferative disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Morra, M. et al. X-linked lymphoproliferative disease: a progressive immunodeficiency. Annu. Rev. Immunol. 19, 657–682 (2001).
Schuster, V. & Kreth, H. W. X-linked lymphoproliferative disease is caused by deficiency of a novel SH2 domain-containing signal transduction adaptor protein. Immunol. Rev. 178, 21–28 (2000).
Sayos, J. et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature 395, 462–469 (1998).
Nichols, K. E. et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl Acad. Sci. USA 95, 13765–13770 (1998).
Coffey, A. J. et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nature Genet. 20, 129–135 (1998).
Wu, C. et al. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nature Immunol. 2, 410–414 (2001).
Cocks, B. G. et al. A novel receptor involved in T cell activation. Nature 376, 260–263 (1995).
Mavaddat, N. et al. Signaling lymphocytic activation molecule (CDw150) is homophilic but self-associates with very low affinity. J. Biol. Chem. 275, 28100–28109 (2000).
Castro, A. G. et al. Molecular and functional characterization of mouse signaling lymphocytic activation molecule (SLAM): differential expression and responsiveness in TH1 and TH2 cells. J. Immunol. 163, 5860–5870 (1999).
Aversa, G., Chang, C. C., Carballido, J. M., Cocks, B. G. & de Vries, J. E. Engagement of the signaling lymphocytic activation molecule (SLAM) on activated T cells results in IL-2-independent, cyclosporin A-sensitive T cell proliferation and IFN-γ production. J. Immunol. 158, 4036–4044 (1997).
Sayos, J. et al. Potential pathways for regulation of NK and T cell responses: differential X-linked lymphoproliferative syndrome gene product SAP interactions with SLAM and 2B4. Int. Immunol. 12, 1749–1757 (2000).
Nakajima, H. et al. Patients with X-linked lymphoproliferative disease have a defect in 2B4 receptor-mediated NK cell cytotoxicity. Eur. J. Immunol. 30, 3309–3318 (2000).
Tangye, S. G., Phillips, J. H., Lanier, L. L. & Nichols, K. E. Functional requirement for SAP in 2B4-mediated activation of human natural killer cells as revealed by the X-linked lymphoproliferative syndrome. J. Immunol. 165, 2932–2936 (2000).
Parolini, S. et al. X-linked lymphoproliferative disease. 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus-infected cells. J. Exp. Med. 192, 337–346 (2000).
Nakajima, H. & Colonna, M. 2B4: an NK cell activating receptor with unique specificity and signal transduction mechanism. Hum. Immunol. 61, 39–43 (2000).
Tangye, S. G. et al. Cutting edge: human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP-2 and the adaptor signaling protein SAP. J. Immunol. 162, 6981–6985 (1999).
Li, S. C. et al. Novel mode of ligand binding by the SH2 domain of the human XLP disease gene product SAP/SH2D1A. Curr. Biol. 9, 1355–1362 (1999).
Poy, F. et al. Crystal structures of the XLP protein SAP reveal a class of SH2 domains with extended, phosphotyrosine-independent sequence recognition. Mol. Cell 4, 555–561 (1999).
Mikhalap, S. V. et al. CDw150 associates with src-homology 2-containing inositol phosphatase and modulates CD95-mediated apoptosis. J. Immunol. 162, 5719–5727 (1999).
Lemay, S., Davidson, D., Latour, S. & Veillette, A. Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol. Cell. Biol. 20, 2743–2754 (2000).
Tamir, I. et al. The RasGAP-binding protein p62dok is a mediator of inhibitory FcγRIIB signals in B cells. Immunity 12, 347–358 (2000).
Suzu, S. et al. p56(dok-2) as a cytokine-inducible inhibitor of cell proliferation and signal transduction. EMBO J. 19, 5114–5122 (2000).
Yamanashi, Y. & Baltimore, D. Identification of the Abl- and rasGAP-associated 62 kD protein as a docking protein, Dok. Cell 88, 205–211 (1997).
Carpino, N. et al. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell 88, 197–204 (1997).
Di Cristofano, A. et al. Molecular cloning and characterization of p56dok-2 defines a new family of RasGAP-binding proteins. J. Biol. Chem. 273, 4827–4830 (1998).
Helgason, C. D. et al. Targeted disruption of SHIP leads to hematopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 12, 1610–1620 (1998).
Cooke, M. P. & Perlmutter, R. M. Expression of a novel form of the fyn proto-oncogene in hematopoietic cells. New Biol. 1, 66–74 (1989).
Davidson, D., Chow, L. M., Fournel, M. & Veillette, A. Differential regulation of T cell antigen responsiveness by isoforms of the src-related tyrosine protein kinase p59fyn. J. Exp. Med. 175, 1483–1492 (1992).
Chow, L. M. & Veillette, A. The Src and Csk families of tyrosine protein kinases in hematopoietic cells. Semin. Immunol. 7, 207–226 (1995).
Stein, P. L., Lee, H. M., Rich, S. & Soriano, P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell 70, 741–750 (1992).
Weiss, A. & Littman, D. R. Signal transduction by lymphocyte antigen receptors. Cell 76, 263–274 (1994).
Liu, Q. et al. The inositol polyphosphate 5-phosphatase ship is a crucial negative regulator of B cell antigen receptor signaling. J. Exp. Med. 188, 1333–1342 (1998).
Yamanashi, Y. et al. Role of the rasGAP-associated docking protein p62(dok) in negative regulation of B cell receptor-mediated signaling. Genes Dev. 14, 11–16 (2000).
Yang, W. C., Collette, Y., Nunes, J. A. & Olive, D. Tec kinases: a family with multiple roles in immunity. Immunity. 12, 373–382 (2000).
Kane, L. P., Andres, P. G., Howland, K. C., Abbas, A. K. & Weiss, A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-γ but not TH2 cytokines. Nature Immunol. 2, 37–44 (2001).
Kane, L. P., Shapiro, V. S., Stokoe, D. & Weiss, A. Induction of NF-κB by the Akt/PKB kinase. Curr. Biol 9, 601–604 (1999).
Miceli, M. C. & Parnes, J. R. The roles of CD4 and CD8 in T cell activation. Semin. Immunol. 3, 133–141 (1991).
Thompson, A. D. et al. EAT-2 is a novel SH2 domain containing protein that is up regulated by Ewing's sarcoma EWS/FLI1 fusion gene. Oncogene 13, 2649–2658 (1996).
Abraham, N., Miceli, M. C., Parnes, J. R. & Veillette, A. Enhancement of T cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature 350, 62–66 (1991).
Latour, S., Fournel, M. & Veillette, A. Regulation of T cell antigen receptor signaling by Syk tyrosine protein kinase. Mol. Cell. Biol. 17, 4434–4441 (1997).
Rubin, L. A., Kurman, C. C., Biddison, W. E., Goldman, N. D. & Nelson, D. L. A monoclonal antibody 7G7/B6, binds to an epitope on the human interleukin-2 (IL-2) receptor that is distinct from that recognized by IL-2 or anti-Tac. Hybridoma 4, 91–102 (1985).
Leo, O., Foo, M., Sachs, D. H., Samelson, L. E. & Bluestone, J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc. Natl. Acad. Sci. USA 84, 1374–1378 (1987).
Wilde, D. B., Marrack, P., Kappler, J., Dialynas, D. P. & Fitch, F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphoctyes lines. J. Immunol. 131, 2178–2183 (1983).
Veillette, A., Bookman, M. A., Horak, E. M. & Bolen, J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell 55, 301–308 (1988).
Gervais, F. G., Chow, L. M., Lee, J. M., Branton, P. E. & Veillette, A. The SH2 domain is required for stable phosphorylation of p56lck at tyrosine 505, the negative regulatory site. Mol. Cell. Biol. 13, 7112–7121 (1993).
Peri, K. G. et al. Interactions of the SH2 domain of lymphocyte-specific tyrosine protein kinase p56lck with phosphotyrosine-containing proteins. Oncogene 8, 2765–2772 (1993).
Acknowledgements
We thank the members of the Veillette laboratory, M. Nemer and A. Fischer for critical comments on the manuscript and I. Gamache and X. Shi for technical help. Supported by grants from the National Cancer Institute of Canada, the CANVAC National Centre of Excellence and the Canadian Institutes of Health Research (to A. V.), the Institut National de la Santé et de la Recherche Médicale and the Association pour la Recherche sur le Cancer (France) (to S. L.) and the National Cancer Institute of Canada (to R. K. H.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Latour, S., Gish, G., Helgason, C. et al. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nat Immunol 2, 681–690 (2001). https://doi.org/10.1038/90615
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/90615
This article is cited by
-
SLAM receptors foster iNKT cell development by reducing TCR signal strength after positive selection
Nature Immunology (2019)
-
Measles virus hemagglutinin triggers intracellular signaling in CD150-expressing dendritic cells and inhibits immune response
Cellular & Molecular Immunology (2016)
-
CTP synthase 1 deficiency in humans reveals its central role in lymphocyte proliferation
Nature (2014)
-
XLP: Clinical Features and Molecular Etiology due to Mutations in SH2D1A Encoding SAP
Journal of Clinical Immunology (2014)
-
Identification of a new isoform of the murine Sh2d1a gene and its functional implications
Science China Life Sciences (2014)