Abstract
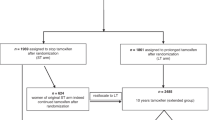
In 1985 a second randomisation was initiated for women in the treatment arm of the Scottish Tamoxifen Trial either to stop tamoxifen at 5 years or to continue indefinitely. A preliminary analysis of outcome in 342 patients at a median follow-up of 6 years suggests that a worthwhile gain in disease control from continuing adjuvant tamoxifen beyond 5 years is unlikely. [Hazard ratio for events (relapse or death without relapse) is 1.27, 95% CI = 0.87 - 1.85.] There is a suggestion that therapy for longer than 5 years may increase the risk of endometrial carcinoma (P = 0.064).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Stewart, H., Forrest, A., Everington, D. et al. Randomised comparison of 5 years of adjuvant tamoxifen with continuous therapy for operable breast cancer. Br J Cancer 74, 297–299 (1996). https://doi.org/10.1038/bjc.1996.356
Issue Date:
DOI: https://doi.org/10.1038/bjc.1996.356
This article is cited by
-
A follow-up study of a randomized controlled study evaluating safety and efficacy of leuprorelin acetate every-3-month depot for 2 versus 3 or more years with tamoxifen for 5 years as adjuvant treatment in premenopausal patients with endocrine-responsive breast cancer
Breast Cancer (2021)
-
Adjuvante endokrine Therapie beim frühen Mammakarzinom
Der Gynäkologe (2018)
-
Adjuvant endocrine monotherapy for postmenopausal early breast cancer patients with hormone-receptor positive: a systemic review and network meta-analysis
Breast Cancer (2018)
-
Extended adjuvant endocrine therapy in early breast cancer: a meta-analysis of published randomized trials
Medical Oncology (2017)
-
A randomized controlled study evaluating safety and efficacy of leuprorelin acetate every-3-months depot for 2 versus 3 or more years with tamoxifen for 5 years as adjuvant treatment in premenopausal patients with endocrine-responsive breast cancer
Breast Cancer (2016)