Abstract
Celiac disease is a disorder of the small intestine caused by an inappropriate immune response to wheat gluten and similar proteins of barley and rye. At present, the only available treatment is a strict gluten-exclusion diet; hence the need for alternative treatments. Recent advances have improved our understanding of the molecular basis for this disorder and there are several attractive targets for new treatments. Oral enzyme supplementation is designed to accelerate gastrointestinal degradation of proline-rich gluten, especially its proteolytically stable antigenic peptides. Complementary strategies aiming to interfere with activation of gluten-reactive T cells include the inhibition of intestinal tissue transglutaminase activity to prevent selective deamidation of gluten peptides, and blocking the binding of gluten peptides to the HLA-DQ2 or HLA-DQ8 molecules. Other possible treatments include cytokine therapy, and selective adhesion molecule inhibitors that interfere with inflammatory reactions, some of which are already showing promise in the clinic for other gastrointestinal diseases.
Similar content being viewed by others
Introduction
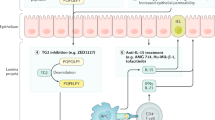
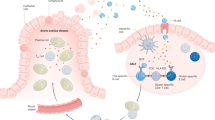
Celiac disease—also known as celiac sprue and GLUTEN-sensitive enteropathy—is a prevalent (∼1:100) food hypersensitivity disorder caused by an inflammatory response to wheat gluten and similar proteins of barley and rye.1 The resulting intestinal inflammation often causes symptoms related to malabsorption, but in many patients extra-intestinal symptoms dominate, and in others the disease is clinically silent. Genes encoding HLA-DQ2 and HLA-DQ8 molecules are the single most important predisposing genetic factor; however, although these polymorphisms are necessary, they are not sufficient for disease development. HLA-DQ2 and HLA-DQ8 predispose to disease development by preferential presentation to mucosal CD4+ T cells of proline-rich gluten peptides that have undergone DEAMIDATION by the enzyme TISSUE TRANSGLUTAMINASE (TRANSGLUTAMINASE 2; TG2). Fewer details are known about the effector mechanisms that lead to the development of the typical celiac lesion—villous atrophy, crypt hyperplasia and infiltration of inflammatory cells (Figure 1)—but, once activated, gluten-reactive CD4+ T cells produce cytokines and are likely to control the inflammatory reactions that produce the celiac lesion. This notion is based on the nature of the HLA association and the unique presentation of gluten antigens to T cells by HLA-DQ2 or HLA-DQ8 in the intestine. Recent advances have improved our understanding of the molecular basis for this disorder,2 and new targets for rational therapy have been identified. This paper reviews concepts for new treatments and their current status.
Factors that contribute to the development of celiac disease and that can be targeted for new therapies are depicted. Proline-rich fragments of gluten that are resistant to processing by luminal and brush-border enzymes survive digestion5 and can be transported across the mucosal epithelium as polypeptides. CD4+ T cells in the lamina propria recognize predominantly deamidated gluten peptides37 in the context of HLA-DQ2 or HLA-DQ8 molecules on the cell surface of antigen-presenting cells (APCs).38 The deamidation of gluten peptides is mediated by tissue transglutaminase (TG2).39,40,41 The gluten-reactive CD4+ T cells produce interferon (IFN)-γ on activation.27 IFN-γ is also produced by T cells in the epithelium.42 Interleukin (IL)-15, produced by either mononuclear cells in the lamina propria or by enterocytes,30,31 stimulates T cells to migrate to the epithelium and facilitate killing of enterocytes by upregulated expression of MIC by enterocytes and NKG2D by intraepithelial T cells.29,32 IL-15 production is stimulated by gluten.28,29 Gluten can also induce production of the intestinal peptide zonulin, which acts on tight junctions and increases epithelial permeability.43 Adapted with permission from2 ©(2002) Macmillan Publishers Ltd.
Current treatment and the need for alternatives
The current treatment for celiac disease is lifelong adherence to a strict gluten-exclusion diet. Gluten is, however, a common (and in many countries unlabeled) ingredient in the human diet, presenting a big challenge for celiac disease patients. Bona fide gluten-free products are not widely available and are usually more expensive than their gluten-containing counterparts. Unsurprisingly, dietary compliance is, at best, imperfect in a large fraction of patients, especially adolescents and adults. There is therefore an urgent need to develop safe and effective therapeutic alternatives.
The quality of life of celiac disease patients would improve if there was a treatment that would allow some gluten to be consumed over a short period of time, for instance during social events or travel. Documentation of the long-term safety for such an agent would probably be less necessary because the indication would be for intermittent treatment. This type of therapy could then pave the way for long-term or permanent treatment.
One goal for a new therapeutic agent would be to enhance the return of full intestinal function in patients who show incomplete recovery in response to a gluten-free diet. This agent might also allow moderate quantities (1–5 g/day) of gluten to be tolerated. Although this is less gluten than is consumed as part of a typical Western diet (∼20 g/day), it could improve quality of life by protecting patients from most forms of 'hidden gluten'. Finally, there is a particular need for treatment alternatives for refractory sprue, which, although rare, is currently treated only with harsh immunosuppressive drugs.
Potential new therapies
Enzyme therapy
Prolyl endopeptidases (PEPs) are endoproteolytic enzymes. In contrast to human gastrointestinal proteases, PEPs can readily cleave proline-rich immunostimulatory gluten peptides.3 The potential to use PEPs as a treatment for celiac disease is appealing because their specificity complements gastrointestinal proteolytic processes. Not only does every PEP-catalyzed cleavage generate one new amino and carboxyl terminus, but it also truncates the long-peptide end products of gastric and duodenal gluten metabolism. Both of these outcomes provide residual smaller peptides that are suitable substrates for the intestinal brush-border aminopeptidases and carboxypeptidases. Microorganisms and plants express various PEPs. There is also a human PEP, but this is expressed only in the cytosol,4 and is therefore unlikely to have a physiological role in the degradation of gluten peptides.
It has been proposed that oral administration of a therapeutic dose of a suitably formulated PEP might counter the toxic effects of moderate quantities of ingested gluten.3 This hypothesis is supported by extensive in vitro, in vivo (in rats) and ex vivo (using biopsy-derived T cells) studies on synthetic gluten peptides, recombinant gliadin molecules and whole gluten as obtained in a grocery store.3,5,6,7,8 In each case, dose-dependent breakdown of immunotoxic gluten peptides by a Flavobacterium meningosepticum PEP was observed. This proteolytic action was also reflected in a concomitant reduction of immunogenicity, as judged by reduced stimulation of most polyclonal T-cell lines from patients with celiac disease.8 Together, these results set the stage for controlled studies in patients with celiac disease. The primary near-term clinical challenge is to identify a threshold dose of gluten that is well tolerated by most patients when consumed in conjunction with a specified PEP dose.
Until recently, most studies directed towards enzyme therapy have utilized the F. meningosepticum PEP. This enzyme meets most criteria for a therapeutically effective PEP, with the possible exception of sluggish kinetics against a few T-cell EPITOPES in gluten. Its primary limitation lies in the high production costs, which are presumably due to its relatively poor level of heterologous expression. A homologous PEP from Myxococcus xanthus seems to be comparable to the F. meningosepticum enzyme with respect to gluten detoxification, but can be expressed at much higher levels in Escherichia coli.7 In addition, Lactobacillus helveticus has a zinc-dependent PEP that can also cleave long substrates with relatively broad subsite specificity.9 Further studies should help to clarify the relative pros and cons of the available PEPs and show which should be used for human clinical trials.
A pharmacologically useful oral PEP formulation might also include one or more complementary enzymes. For example, the cysteine endoprotease EPB derived from barley is a glutamine-specific enzyme that rapidly hydrolyzes intact gliadin polypeptides (Box 1) into short peptides under acidic conditions.10 Although it is unable to cleave some of the more proline-rich inflammatory peptides, such as the 33-mer from α-gliadin, it could enhance the efficacy of a duodenally active PEP by simplifying gluten structure and texture while food is still in the stomach.
The long-term safety of a candidate therapeutic enzyme (or enzyme cocktail) designed to act in the upper small intestine must also be evaluated, initially in animals and subsequently also in humans. Primary safety concerns must ensure that the exogenous enzyme is non-allergenic, and is not assimilated intact into the bloodstream in appreciable quantities. Other potential risks include structural damage to intestinal mucosa and the consequent loss of nutrient absorptive capacity or alterations in regulation of gut hormones. In this regard, it must be noted that some proteases of pharmacological interest (e.g. barley EPB) have been components of the human diet for a long time, and are therefore unlikely to present a health risk.
Proteases can also detoxify gluten-containing products before they are ingested. This is particularly relevant for products that have minor, but significant, gluten content. Analogously, proteases of certain lactobacilli present in sourdough are able to proteolyze proline-rich gluten peptides,11 and a challenge experiment indicates that celiac disease patients, at least in the short term, tolerate a carefully prepared sourdough bread.12
Tissue transglutaminase inhibitors
The importance of gluten-peptide deamidation for the recognition of T-cell epitopes implicates TG2 inhibitors as a candidate therapy for celiac disease. Indeed, ex vivo gluten challenge of patient-derived small-intestine biopsies showed that the proliferative capacity of gluten-responsive T cells could be blocked by co-treatment with cystamine, a TG2 inhibitor.13
The efficacy and side effects of TG2 inhibitors as a treatment of celiac disease are unknown. A few gluten T-cell epitopes are recognized without being modified by TG2.14,15 Activation of such cells might be sufficient to drive the gut inflammation. Although TG2 knockout mice have no overt spontaneous abnormalities,16,17 they develop splenomegaly, autoantibodies and immune complex glomerulonephritis by systemic triggering of apoptosis.18 Thus, the optimal TG2 inhibitor should have activity limited to the gut mucosa. Local side effects related to extracellular matrix formation and wound healing can be envisaged. Of course, a prerequisite for investigating safety and efficacy is the availability of one or more selective TG2 inhibitors.
Although several TG2 inhibitors have been described in the literature, many of these compounds (e.g. monodansyl cadaverine) contain primary amines in addition to potential inhibitory motifs, and it remains unclear whether the observed effects are due to excess competing amines or blockage of TG2 substrate turnover. A few studies have utilized a suicide inhibitor, L-682777, which inhibits human TG2, however, L-682777 is a specific inhibitor of Factor XIIIa, and is therefore unsuitable for evaluating TG2 biology in animals and humans.
More recently, mechanism-based active-site inhibitors of guinea pig and human TG2 have been reported. Synthetic compounds containing halo-dihydroisoxazole are particularly promising. One such compound, KCC009, inhibits intestinal TG2 when dosed orally, and is well tolerated by rodents at pharmacologically effective doses.19 It also has a short serum half-life, which limits the exposure of other organs to this compound. Such an inhibitor could be used to validate TG2 as an appropriate therapeutic target for treating celiac disease.
Blocking of HLA-DQ peptide presentation
The crucial role of the HLA in celiac disease development makes it an obvious target for therapeutic intervention. Blocking the binding sites of HLA-DQ2 and, to a lesser extent, HLA-DQ8, would prevent the presentation of disease-inducing gluten peptides.
The concept of HLA blockade is not new and was developed without much success for the treatment of type 1 diabetes and rheumatoid arthritis. The lack of success was due partly to difficulties in obtaining effective drug delivery. This should be less of a problem in celiac disease because the blocking compound can be administered before or in parallel with the antigen (i.e. gluten). In our opinion, HLA blockade as a therapy for celiac disease should be pursued.
Side effects, such as immunosuppression, are unlikely because many healthy individuals are homozygous for HLA alleles. The recently solved X-ray crystal structure of HLA-DQ2 complexed with a deamidated gluten peptide provides important information for the development of an HLA-DQ2-blocking compound.20 A blocking compound should prevent presentation of gluten peptides to T cells and be unrecognizable by any T cell, to avoid hypersensitivity responses to the blocking compound itself. This might be achieved by making the compound either small enough to avoid forming interactions with the T-cell receptor, or so big that no T-cell receptor can dock onto HLA-DQ2 with the bound blocker.
Silencing of gluten-reactive T cells
Celiac disease is exceptional in that the disease can be put in remission and induced in a controlled fashion. This could be utilized for therapeutic purposes. Conceivably, gluten-reactive T cells could be eliminated or made unresponsive by oral gluten challenge concomitant with the administration of agents that alter the outcome of the T-cell activation. Antibodies to CD321 and CD154 (CD40L),22 for example, can induce T-cell silencing, but they produce unwanted side effects such as toxic cytokine syndrome (anti-CD3) and thromboembolic events (anti-CD154). Alternatively, gluten-reactive T cells might be silenced by soluble dimers of HLA–peptide complexes, because such dimers induce the apoptosis of antigen-specific T cells as a result of inappropriate stimulation.23 This approach is complicated by an increasing number of characterized gluten epitopes. Induction of tolerance by intranasal administration of gluten or gluten T-cell epitopes has also been suggested as a possible treatment modality.24 Moreover, tolerance can be achieved by targeting gluten epitopes to dendritic cells that induce T-cell tolerance, but this approach is further complicated by the presence of multiple T-cell epitopes. In addition, it is unclear which marker should be used to target tolerogenic dentritic cells in the intestinal mucosa.
Cytokine therapy
Various cytokine therapies are being developed for the treatment of chronic inflammatory diseases. Celiac disease is not a pioneering disorder for the testing of such therapeutics, owing to the low acceptance of side effects by patients and the presumed lower consumer market. Nevertheless, this approach might yield a compound that is well tolerated with a mode of action relevant to celiac disease. There are already some compounds that merit attention.
Interleukin (IL)-10 counterbalances the TH1 IMMUNE RESPONSE that is typical of celiac disease. It can suppress gluten-dependent T-cell activation in ex-vivo cultured celiac intestinal mucosa,25 but, in a pilot study of refractory celiac disease, recombinant IL-10 had little effect.26 IL-10 has also been a candidate therapy for Crohn's disease but, notably, a Phase II/III trial with recombinant IL-10 was discontinued owing to a lack of effect (Table 1).
Interferon (IFN)-γ is the dominant cytokine produced by gluten-reactive T cells,27 and antibodies neutralizing IFN-γ should have a good chance of curtailing the inflammatory effects of the anti-gluten T-cell response. Antibodies of this kind are being tested in Phase I/II trials for Crohn's disease (Table 1). Depending on the results of these trials, anti-IFN-γ agents might become candidates for testing in celiac disease.
IL-15 is thought to be the central mediator of the innate effects of gluten in celiac disease,28,29,30,31 making IL-15 neutralizing agents therapeutic candidates. Two such compounds are in clinical trials; a humanized anti-IL-15 antibody, HuMax-IL-15, is in Phase II trials for rheumatoid arthritis and possibly other inflammatory conditions, and an IL-15/Fc chimeric protein, CRB-15, is in preclinical testing (Table 1).29 Earlier studies suggest that HuMax-IL-15 has acceptable side effects, and might therefore be a candidate for testing in celiac disease.
Selective adhesion molecule inhibition
Another novel class of therapeutic agents under development for the treatment of chronic inflammatory diseases acts by selective inhibition of leukocyte adhesion. This inhibition prevents leukocytes from migrating into inflamed tissues. For example, a humanized antibody against INTEGRIN-α4, natalizumab, is being used for the treatment of multiple sclerosis, and is under evaluation for inflammatory bowel disease (IBD) (Table 1). A small-molecule integrin-α4 antagonist, T0047, has also been developed and is undergoing clinical testing. Preliminary reports indicate that natalizumab has beneficial effects in IBD with moderate side effects.
Another humanized monoclonal antibody, MLN02, which targets the adhesion molecule integrin-α4β7 that is expressed by gut T cells, is being tested in Phase II clinical trials for the treatment of IBD. This agent is intriguing with respect to celiac disease, because it aims to prevent migration of T cells to the lamina propria. Conceivably, this agent could interfere with the action of the HLA-DQ-restricted T cells in the lamina-propria-recognizing gluten peptides. Possible side effects include increased susceptibility to gastrointestinal infections and a potential for disturbances in oral tolerance to food proteins.
Other therapeutic options
The recent finding that NKG2D is important for the killing of enterocytes by intraepithelial lymphocytes in celiac disease led to the suggestion that NKG2D antagonists might be used for therapy.29,32 This concept has gained support from a mouse model of type 1 diabetes. Treatment with a nondepleting anti-NKG2D monoclonal antibody during the prediabetic stage in NOD MICE prevented disease completely by impairing the expansion and function of autoreactive CD8+ T cells.33 ZONULIN antagonists have also been suggested as therapy for celiac disease aiming to prevent gluten-induced disruption of the epithelial barrier.34
Finally, the development of grains that have low or no content of immunotoxic sequences, but with reasonable baking quality, should be mentioned. Such grains can potentially be developed by selective breeding of ancient wheat varieties,35 by transgenic technology involving mutation of sequences giving rise to immunostimulatory sequences,36 by small interfering RNA (siRNA) technology, or by incorporation of nontoxic gluten genes into harmless organisms such as rice. Although these grains would be technically challenging to engineer, and there is a possibility that cross-pollination with gluten-containing grains might lead to reintroduction of immunotoxic sequences, the availability of such grains could give patients with celiac disease a nutritionally better diet. If used for the nutritional needs of the general population, this could eventually prevent celiac disease.
The way forward
Criteria for a clinical candidate
Notwithstanding promising progress, an animal model for celiac disease remains elusive. In its absence, the evaluation of preclinical efficacy of an experimental therapeutic agent for celiac disease is limited to in vitro studies and, in some cases, pharmacodynamic measurements in healthy humans. In addition, regardless of its mode of action, a clinical candidate for treating celiac disease must satisfy certain safety criteria that can be tested in animals. These include good oral and systemic tolerance of the agent, absence of antigenicity, and localization of the drug to the gut. In particular, structural integrity should be confirmed via post-mortem histological evaluations of the entire gastrointestinal tract of treated animals.
As objective endpoints for early clinical trials in patients with celiac disease can probably be achieved in less than 1 month (see below), a 3–6 month initial toxicological evaluation of an experimental therapeutic would be appropriate. Downstream clinical studies would require long-term safety testing in animals; the criteria for such studies would be different when drugs are intended for intermittent therapy to when intended for regular, lifelong use.
Monitoring the treatment effect
The strategies used for early clinical studies (phase I and II) with an experimental therapeutic agent for celiac disease are of particular interest. If well designed, these studies can yield pivotal efficacy data, while concomitantly identifying the subpopulation of patients that stand to benefit the most from the new drug. At the same time, because health risks are usually at their highest when studying a new chemical entity, such studies must be conducted with particular caution.
No efforts have been made thus far to evaluate the efficacy of an investigational drug for celiac disease, so the question of how best to perform early proof-of-concept clinical trials remains unaddressed. Clearly the most effective format for such clinical trials would be a crossover mode in which patients with celiac disease are exposed alternately to gluten plus placebo and gluten plus drug, for 2–4 weeks each. The variables in such a format would be gluten dose, duration of gluten exposure, and evaluation methods at the beginning and end of each stage of the trial.
Ordinarily, gastroenterologists use serum antibody levels and endoscopic and intestinal histological analyses for evaluating patients with celiac disease. Although their utility for initial diagnosis as well as follow-up care of patients is well documented, endoscopic and histological methods have serious limitations in the context of evaluating investigational drugs.
The conversion from normal to abnormal antibody titers requires exposure to high levels of gluten for several months, which might produce disturbing symptoms and be a health risk. By contrast, whereas endoscopic and histological procedures are more sensitive and specific, repeated endoscopic evaluations in the context of a crossover clinical trial involve some risk, are expensive, and require experienced evaluators to extract relatively subjective data. An alternative non-invasive approach is the use of functional tests of gut malfunction (e.g. xylose absorption tests, fecal fat analysis, lactulose/mannitol permeability tests), which are sensitive but non-specific tests for celiac disease. Finally, the radiotelemetry video capsule might also provide useful information for assessment of overall small-intestinal structure in the context of a clinical trial.
For some therapeutic approaches, such as enzyme therapy, it might be possible to assess pharmacodynamic efficacy in healthy adult volunteers, because the incremental benefit of an oral enzyme formulation on gastric and/or duodenal proteolysis of gluten can be safely monitored by intestinal intubation with small-diameter plastic tubes. Similarly, the efficacy of certain other therapeutic strategies, such as cytokine or integrin therapy, is likely to be validated in patients with more serious inflammatory bowel disorders before initiating clinical trials for their use as a treatment for celiac disease.
Conclusions
The need for alternatives to the gluten-exclusion diet for the treatment of celiac disease has been made even more urgent by the increasing number of patients diagnosed with this disease. Fundamental studies have revealed several attractive targets for therapy, some of which are already showing promise in the context of other medical conditions. It will be interesting to see whether any of these will become reality in the coming years.
Review criteria
PubMed was searched in November 2004 using the terms “celiac disease”, “therapy”, “prolyl AND peptidase”, “transglutaminase”, “integrin”, “interferon” and “interleukin”. In addition, searches were done using SciFinder Scholar, Google, Google Scholar and the Investigational Drugs Database. Corporate websites of companies known to be developing relevant anti-inflammatory drugs were also searched. Citations were chosen based on their direct relevance to statements in the text. Some information cited in the text has been presented only at meetings and symposia; in such situations the meeting abstract is cited.
References
Mäki M et al. (2003) Prevalence of celiac disease among children in Finland. N Engl J Med 348: 2517–2524
Sollid LM (2002) Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol 2: 647–655
Hausch F et al. (2002) Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol 283: G996–G1003
Vanhoof G et al. (1994) Cloning and sequence analysis of the gene encoding human lymphocyte prolyl endopeptidase. Gene 149: 363–366
Shan L et al. (2002) Structural basis for gluten intolerance in celiac sprue. Science 297: 2275–2279
Piper JL et al. (2004) Effect of prolyl endopeptidase on digestive-resistant gliadin peptides in vivo. J Pharmacol Exp Ther 311: 213–219
Shan L et al. (2004) Comparative biochemical analysis of three bacterial prolyl endopeptidases: implications for coeliac sprue. Biochem J 383: 311–318
Marti T et al. (2005) Prolyl endopeptidase-mediated destruction of T cell epitopes in whole gluten: chemical and immunological characterization. J Pharmacol Exp Ther 312: 19–26
Chen YS et al. (2003) Identification and characterization of Lactobacillus helveticus PepO2, an endopeptidase with post-proline specificity. Appl Environ Microbiol 69: 1276–1282
Zhang N and Jones BL (1996) Purification and partial characterization of a 31-kDa cysteine endopeptidase from germinated barley. Planta 199: 565–572
Di Cagno R et al. (2002) Proteolysis by sourdough lactic acid bacteria: effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Appl Environ Microbiol 68: 623–633
Di Cagno R et al. (2004) Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Appl Environ Microbiol 70: 1088–1096
Molberg Ø et al. (2001) T cells from celiac disease lesions recognize gliadin epitopes deamidated in situ by endogenous tissue transglutaminase. Eur J Immunol 31: 1317–1323
Vader W et al. (2002) The gluten response in children with celiac disease is directed toward multiple gliadin and glutenin peptides. Gastroenterology 122: 1729–1737
Arentz-Hansen H et al. (2002) Celiac lesion T cells recognize epitopes that cluster in regions of gliadins rich in proline residues. Gastroenterology 123: 803–809
De Laurenzi V and Melino G (2001) Gene disruption of tissue transglutaminase. Mol Cell Biol 21: 148–155
Nanda N et al. (2001) Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem 276: 20673–20678
Szondy Z et al. (2003) Transglutaminase 2−/− mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc Natl Acad Sci USA 100: 7812–7817
Choi et al. (2005) Chemistry and biology of dihydroisoxazole derivatives: selective inhibitors of human transglutaminase 2. Chem. Biol., in press
Kim CY et al. (2004) Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. Proc Natl Acad Sci USA 101: 4175–4179
Chatenoud L (2003) CD3-specific antibody-induced active tolerance: from bench to bedside. Nat Rev Immunol 3: 123–132
Burkly LC (2001) CD40 pathway blockade as an approach to immunotherapy. Adv Exp Med Biol 489: 135–152
Appel H et al. (2001) Anergy induction by dimeric TCR ligands. J Immunol 166: 5279–5285.
Maurano F et al. (2001) Intranasal administration of one alpha gliadin can downregulate the immune response to whole gliadin in mice. Scand J Immunol 53: 290–295
Salvati VM et al. (2005) Recombinant human IL-10 suppresses gliadin dependent T cell activation in ex vivo cultured celiac intestinal mucosa. Gut 54: 46–53
Mulder CJ et al. (2001) A pilot study of recombinant human interleukin-10 in adults with refractory coeliac disease. Eur J Gastroenterol Hepatol 13: 1183–1188
Nilsen EM et al. (1995) Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon gamma. Gut 37: 766–776
Maiuri L et al. (2003) Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet 362: 30–37
Hüe S et al. (2004) A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 21: 367–377
Maiuri L et al. (2000) Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology 119: 996–1006
Mention JJ et al. (2003) Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology 125: 730–745
Meresse B et al. (2004) Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 21: 357–366
Ogasawara K et al. (2004) NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity 20: 757–767
Fasano A et al. (2000) Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 355: 1518–1519
Molberg Ø et al. Mapping of gluten T cell epitopes in the bread wheat ancestors; implications for celiac disease. Gastroenterology, in press
Vader LW et al. (2003) Characterization of cereal toxicity for celiac disease patients based on protein homology in grains. Gastroenterology 125: 1105–1113
Sjöström H et al. (1998) Identification of a gliadin T-cell epitope in coeliac disease: general importance of gliadin deamidation for intestinal T-cell recognition. Scand J Immunol 48: 111–115
Lundin KE et al. (1993) Gliadin-specific, HLA-DQ(α1*0501,β1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J Exp Med 178: 187–196
Molberg Ø et al. (1998) Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med 4: 713–717
van de Wal Y et al. (1998) Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J Immunol 161: 1585–1588
Vader LW et al. (2002) Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J Exp Med 195: 643–649
Forsberg G et al. (2002) Paradoxical coexpression of proinflammatory and down-regulatory cytokines in intestinal T cells in childhood celiac disease. Gastroenterology 123: 667–678
Clemente MG et al. (2003) Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut 52: 218–223
Colombel JF et al. (2001) Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn's disease. Gut 49: 42–46
Yasuda H and Wen L (2004) Prevention of autoimmune diabetes by dendritic cells treated with IL-10 [abstract 358-OR]. Diabetes 53
Villadsen LS et al. (2002) HuMax-IL15 reduces the severity of psoriasis in human skin grafts transplanted on to SCID mice. Brit J Dermatol 147: 1064
Ferrari-Lacraz S et al. (2004) Targeting IL-15 receptor-bearing cells with an antagonist mutant IL-15/Fc protein prevents disease development and progression in murine collagen-induced arthritis. J Immunol 173: 5818–5826
Hommes et al. (2004) Fontolizumab (HuZAF), a humanized anti-IFN-gamma antibody, has clinical activity and excellent tolerability in moderate to severe Crohn's disease. Gastroenterology 127: 332
Arulanandam T (2004) Biological characterisitics of anti-alpha4 intergrin monoclonal antibody (natalizumab) a selective adhesion molecule (SAM) inhibitor for the treatment of multiple sclerosis and Crohn's disease [SA23]. Inflamm Res 53: 3
Feagan B et al. (2003) A randomized, double-blind, placebo-controlled trial of iv MLN-02 in 181 patients with moderately active ulcerative colitis is reported. Am J Gastroenterol 98: s248–s249
Fasano A (2002) Peptide antagonists of zonulin and methods for use of the same. US patent 6,548,925
Acknowledgements
Work by the authors was supported in part by grants from the Research Council of Norway, the NIH and the European Commission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Ludvig M Sollid and Chaitan Khosla are involved in non-profit organizations that develop new treatments for celiac disease. Chaitan Khosla is the President of the Celiac Sprue Research Foundation, and Ludvig M Sollid is a member of the scientific advisory boards of the Celiac Sprue Research Foundation and the National Foundation for Celiac Awareness.
Glossary
- GLUTEN
-
Wheat gluten remains after washing dough and consists of a complex mixture of many gliadin and glutenin polypeptides; gluten-like proteins are also found in rye and barley
- HLA-DQ2 and HLA-DQ8
-
HLA-DQ2 (DQA1*05/DQB1*02) and HLA-DQ8 (DQA1*03/DQB1*0302) are HLA (human leukocyte antigen) molecules associated with celiac disease
- DEAMIDATION
-
The modification of glutamine residues in peptides and proteins to glutamate, or asparagine residues to aspartate
- TISSUE TRANSGLUTAMINASE (TRANSGLUTAMINASE 2; TG2)
-
An enzyme responsible for modifying proteins/peptides by transamidation/deamidation of specific glutamine residues; patients with celiac disease have IgA and IgG antibodies against TG2
- MIC
-
MHC class-I like molecule expressed on gut epithelium; MIC is a ligand for NKG2D
- NKG2D
-
Activating receptor of natural killer cells and CD8+ T cells; NKG2D is expressed on most intraepithelial lymphocytes
- EPITOPE
-
A site on an antigen that is recognized by an antigen receptor (i.e. antibody or T-cell receptor)
- TH1 IMMUNE RESPONSE
-
A type of CD4+ T-cell immune response characterized primarily by the production of interferon-γ
- mutIL15-Fc
-
A lytic and antagonistic IL-15 mutant/Fcgamma2a fusion protein that targets interleukin-15 receptor
- INTEGRIN
-
A cell-surface protein that is involved in cell–cell and cell–matrix interactions
- NOD MICE
-
A mouse strain (nonobese diabetic) that spontaneously develops autoimmune diabetes
- ZONULIN
-
A protein for which the gene is not yet cloned that regulates the permeability of the intestine
Rights and permissions
About this article
Cite this article
Sollid, L., Khosla, C. Future therapeutic options for celiac disease. Nat Rev Gastroenterol Hepatol 2, 140–147 (2005). https://doi.org/10.1038/ncpgasthep0111
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpgasthep0111
This article is cited by
-
The less genetic diversity of Triticeae A and D genomes, the smaller diversity in γ-prolamins CD-epitopes
Genetic Resources and Crop Evolution (2018)
-
Significant Hydrolysis of Wheat Gliadin by Bacillus tequilensis (10bT/HQ223107): a Pilot Study
Probiotics and Antimicrobial Proteins (2018)
-
S9A Serine Protease Engender Antigenic Gluten Catabolic Competence to the Human Gut Microbe
Indian Journal of Microbiology (2018)
-
Prolyl-specific peptidases for applications in food protein hydrolysis
Applied Microbiology and Biotechnology (2015)
-
Alternative Approaches Towards Gluten-Free Dough Development: Recent Trends
Food Engineering Reviews (2014)