Abstract
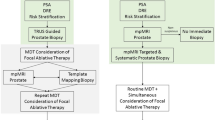
The growing interest in high-intensity focused ultrasound (HIFU) technology is mainly due to its many potential applications as a minimally invasive therapy. It has been introduced to urologic oncology as a treatment for prostate and kidney cancers. While its application in the kidney is still at the clinical feasibility phase, HIFU technology is currently used in daily practice in Europe for the treatment of prostate cancer. Literature describing the results of HIFU for prostate cancer is mainly based on several series of patients from clinical development teams. The latest published results suggest that HIFU treatment is a valuable option for well-differentiated and moderately-differentiated tumors, as well as for local recurrence after external-beam radiation therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Blana A et al. (2004) High-intensity focused ultrasound for the treatment of localized prostate cancer: 5-year experience. Urol 63: 297–300
Poissonnier L et al. (2003) Results of transrectal focused ultrasound for the treatment of localized prostate cancer (120 patients with PSA<or +10 ng/ml). Prog Urol 13: 60–72
Lynn JG and Putman TJ (1944) Histological and cerebral lesions produced by focused ultrasound. Am J Pathol 20: 637–649
ter Haar G (2000) Intervention and therapy. Ultrasound Med Biol 23 (Suppl 1): S51–S54
Chapelon JY et al. (1992) Effects of high-energy focused ultrasound on kidney tissue in the rat and the dog. Eur Urol 22: 147–152
Uchida T et al. (2002) Transrectal high-intensity focused ultrasound for treatment of patients with stage T1b-2n0m0 localized prostate cancer: a preliminary report. Urology 59: 394–398
Chapelon JY et al. (2000) New piezoelectric transducers for therapeutic ultrasound. Ultrasound Med Biol 26: 153–159
Curiel L et al. (2002) 1.5-D high intensity focused ultrasound array for non-invasive prostate cancer surgery. IEEE Trans Ultrason Ferroelectr Freq Control 49: 231–242
Tan JS et al. (2000) Design of focused ultrasound phased arrays for prostate treatment, Ultrasonics Symp Proc IEEE 2: 1247–1251
Chavrier F et al. (2000) Modeling of high intensity focused ultrasound-induced lesions in the presence of cavitation bubbles. J Acoust Soc Am 108: 432–440
Damianou CA et al. (1995) Evaluation of accuracy of a theoretical model for predicting the necrosed tissue volume during focused utrasound surgery. IEEE Trans Ultrason Ferroelec Freq Control 42: 182–187
Curiel L et al. (2004) Experimental evaluation of lesion prediction modelling in the presence of cavitation bubbles: intended for high-intensity focused ultrasound prostate treatment Med Biol Eng Comput 42: 44–54
Hynynen K et al. (1996) A clinical, noninvasive, MR imaging-monitored ultrasound surgery method. Radiographics 16: 185–195
Damianou C et al. (2004) High intensity focused ultrasound ablation of kidney guided by MRI. Ultrasound Med Biol 30: 397–404
Wu T et al. (2001) Assessment of thermal tissue ablation with MR elastography. Magn Reson Med 45: 80–87
Vaezy S et al. (2001) Real-time visualization of high-intensity focused ultrasound treatment using ultrasound imaging. Ultrasound Med Biol 27: 33–42
Souchon R et al. (2003) Visualisation of HIFU lesions using elastography of the human prostate in vivo: preliminary results. Ultrasound Med Biol 29: 1007–1015
Sedelaar JPM et al. (2000): The application of three-dimensional contrast-enhanced ultrasound to measure volume of affected tissue after HIFU treatment for localized prostate cancer. Eur Urol 37: 559–568
Lu J et al. (1996) In vitro measurement of speed of sound during coagulate tissue heating. Ultrasonics Symp Proc IEEE 2: 1299–1302
Kishi M et al. (1975) Experimental studies of effects of intense ultrasound on implantable murine glioma. In: Proceedings of the 2nd European Congress on Ultrasonics in Medicine: 28–33 (Eds. Kazmer E et al.) Amsterdam: Excerpta Medica
Fry FJ and Johnson LK (1978) Tumour irradiation with intense ultrasound. Ultrasound Med Biol 4: 337–341
Moore WE et al. (1989) Evaluation of high-intensity therapeutic ultrasound irradiation in the treatment of experimental hepatoma. J Pediatr Surg 24: 30–33
Yang R et al. (1991) High-intensity focused ultrasound in the treatment of experimental liver cancer. Arch Surg 126: 1010–1010
Chapelon JY et al. (1992) In vivo effects of high-intensity ultrasound on prostatic adenocarcinoma Dunning R3327. Cancer Res 52: 6353–6357
Oosterhof GO et al. (1997) Influence of high-intensity focused ultrasound on the development of metastases. Eur Urol 32: 91–95
Adams JB et al. (1996) High-intensity focused ultrasound ablation of rabbit kidney tumors. J Endourol 10: 71–75
Watkin NA et al. (1997) High-intensity focused ultrasound ablation of the kidney in a large animal model. J Endourol 11: 191–196
Kohrmann KU et al. (2002) Technical characterization of an ultrasound source for noninvasive thermoablation by high-intensity focused ultrasound. BJU Int 90: 248–252
Damianou C (2003) In vitro and in vivo ablation of porcine renal tissues using high-intensity focused ultrasound. Ultrasound Med Biol 29: 1321–1330
Foster RS et al. (1993) Production of prostatic lesions in canines using transrectally administered high-intensity focused ultrasound. Eur Urol 23: 330–336
Gelet A et al. (1993) Prostatic tissue destruction by high-intensity focused ultrasound: experimentation on canine prostate. J Endourol 7: 249–253
Vallancien G et al. (1991) Focused extracorporeal pyrotherapy: experimental results. Eur Urol 20: 211–219
Vallancien G et al. (1993) Focused extracorporeal pyrotherapy: experimental study and feasibility in man. Semin Urol 11: 7–9
Kohrmann KU et al. (2002) High intensity focused ultrasound as noninvasive therapy for multilocal renal cell carcinoma: case study and review of the literature. J Urol 167: 2397–2403
Wu F et al. (2003) Preliminary experience using high intensity focused ultrasound for the treatment of patients with advanced stage renal malignancy. J Urol 170 (Pt 1): 2237–2240
Vallancien G et al. (2004) Transrectal focused ultrasound combined with transurethral resection of the prostate for the treatment of localized prostate cancer: feasibility study. J Urol 171 (Pt 1): 2265–2267
Sanghvi NT et al. (2003) High-intensity focused ultrasound (HIFU) treatment of BPH: results of a multicenter phase III study. Ultrasound Med Biol 29: S102.
Thüroff S et al. (2003) High-intensity focused ultrasound and localized prostate cancer: efficacy results from the European multicentric study. J Endourol 17: 673–677
Rebillard X et al. (2003) Treatment by HIFU of prostate cancer: survey of literature and treatment indications. Prog Urol 13: 1428–1456
Beerlage HP et al. (1999) High-intensity focused ultrasound (HIFU) followed after one to two weeks by radical retropubic prostatectomy: results of a prospective study. Prostate 39: 41–46
Rouviere O et al. (2001) MRI appearance of prostate following transrectal HIFU ablation of localized cancer. Eur Urol 40: 265–274
Chaussy C and Thüroff S (2003) The status of high-intensity focused ultrasound in the treatment of localized prostate cancer and the impact of a combined resection. Curr Urol Rep 4: 248–252
Chaussy CG and Thüroff S (2000) High-intensive focused ultrasound in localized prostate cancer. J Endourol 14: 293–299
Thüroff S and Chaussy C (2000) High-intensity focused ultrasound: complications and adverse events. Mol Urol 4: 183–187
Gelet A et al. (2004) Local recurrence of prostate cancer after external beam radiotherapy: early experience of salvage therapy using high-intensity focused ultrasonography. Urol 63: 625–629
Kupelian PA et al. (2002) Comparison of the efficacy of local therapies for localized prostate cancer in the prostate-specific antigen era: a large single-institution experience with radical prostatectomy and external-beam radiotherapy. J Clin Oncol 20: 3376–3385
Kuban DA et al. (2003) Long-term multi-institutional analysis of stage T1-T2 prostate cancer treated with radiotherapy in the PSA era. Int J Radiat Oncol Biol Phys 57: 915–928
Potters L et al. (2004) Monotherapy for stage T1-T2 prostate cancer: radical prostatectomy, external beam radiotherapy, or permanent seed implantation. Radiother Oncol 71: 29–33
Dearnaley DP et al. (2005) Phase III pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects. Br J Cancer 92: 488–498
Touma NJ et al. (2005) Current status of local salvage therapies following radiation failure for prostate cancer. J Urol 173: 373–379
Acknowledgements
The authors wish to acknowledge Jean-Yves Chapelon PhD and Laura Curiel PhD from the INSERM U556 for their assistance in the description of technological aspects of HIFU.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Albert Gelet is a Clinical Investigator for the Ablatherm® device (EDAP TMS SA, Vaulx-en-Velin, France) and has received support from EDAP TMS SA to attend symposia.
Glossary
- CAVITATION
-
Formation, growth and implosive collapse of bubbles in a liquid irradiated with high-intensity ultrasound resulting in intense local heating
- ABSORPTION COEFFICIENT
-
A measure of the acoustic energy converted into heat in the tissues
- THERMAL DOSE
-
Equivalent time in seconds for an exposure at a reference temperature of 43°C
- SONICATON PARAMETERS
-
Physical properties whose values determine the behavior of the sound wave applied to tissues
- OPERATING FREQUENCY
-
Number of complete oscillations per second of the sound wave generated by the transducer
- PIEZOELECTRIC MATERIALS
-
Crystals that suffer a mechanical deformation when subjected to an electric potential
- POWER DENSITY
-
Power per unit area normal to the direction of propagation
- ACOUSTIC INTENSITY
-
The mean acoustic power transmitted through a unit area
- DURATION of EXPOSURE
-
Time during which the sound wave is applied to the tissues at each burst of high-intensity focused ultrasound
- ON/OFF RATIO
-
Quotient of exposure duration and waiting time between bursts of high-intensity focused ultrasound
- DUNNING R3327
-
Experimental model of adenocarcinoma of the prostate in Copenhagen rats
- AT2, AT6
-
Hormone-independent sublines of Dunning R3327
- NADIR PSA
-
Lowest level of prostate-specific antigen achieved after treatment
- GLEASON SCORE
-
Sum of grades assigned to the two largest cancerous areas of tissue samples; grades range from 1 (least aggressive) to 5 (most aggressive)
- STRESS INCONTINENCE
-
Impaired urethral sphincter function leads to involuntary leakage of urine during physical exertion with increased intra-abdominal pressure
- RESECTION CHIPS
-
Small prostate tissue pieces resulting from transurethral resection of the prostate
- SECONDARY INFRAVESICAL OBSTRUCTION
-
Strictures of the bladder neck or of the urethra after a prostate treatment procedure
Rights and permissions
About this article
Cite this article
Chaussy, C., Thüroff, S., Rebillard, X. et al. Technology Insight: high-intensity focused ultrasound for urologic cancers. Nat Rev Urol 2, 191–198 (2005). https://doi.org/10.1038/ncpuro0150
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpuro0150
This article is cited by
-
Trans-cranial focused ultrasound without hair shaving: feasibility study in an ex vivo cadaver model
Journal of Therapeutic Ultrasound (2014)
-
Eerste ervaringen prostaatkankerbehandeling met High Intensity Focused Ultrasound (HIFU) in Nederland
Tijdschrift voor Urologie (2012)
-
Robotic High-intensity Focused Ultrasound for Prostate Cancer: What Have We Learned in 15 Years of Clinical Use?
Current Urology Reports (2011)