Abstract
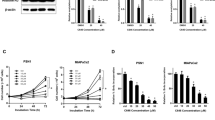
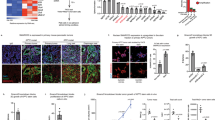
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal human cancers and shows resistance to any therapeutic strategy used. Here we tested small-molecule inhibitors targeting chromatin regulators as possible therapeutic agents in PDAC. We show that JQ1, an inhibitor of the bromodomain and extraterminal (BET) family of proteins, suppresses PDAC development in mice by inhibiting both MYC activity and inflammatory signals. The histone deacetylase (HDAC) inhibitor SAHA synergizes with JQ1 to augment cell death and more potently suppress advanced PDAC. Finally, using a CRISPR-Cas9–based method for gene editing directly in the mouse adult pancreas, we show that de-repression of p57 (also known as KIP2 or CDKN1C) upon combined BET and HDAC inhibition is required for the induction of combination therapy–induced cell death in PDAC. SAHA is approved for human use, and molecules similar to JQ1 are being tested in clinical trials. Thus, these studies identify a promising epigenetic-based therapeutic strategy that may be rapidly implemented in fatal human tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rahib, L. et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74, 2913–2921 (2014).
Hezel, A.F., Kimmelman, A.C., Stanger, B.Z., Bardeesy, N. & Depinho, R.A. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 20, 1218–1249 (2006).
Ghaneh, P., Costello, E. & Neoptolemos, J.P. Biology and management of pancreatic cancer. Gut 56, 1134–1152 (2007).
Maitra, A. & Hruban, R.H. Pancreatic cancer. Annu. Rev. Pathol. 3, 157–188 (2008).
Ryan, D.P., Hong, T.S. & Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 371, 1039–1049 (2014).
Collins, M.A. & Pasca di Magliano, M. Kras as a key oncogene and therapeutic target in pancreatic cancer. Frontiers Physiol. 4, 407 (2013).
Infante, J.R. et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur. J. Cancer 50, 2072–2081 (2014).
Bardeesy, N. & DePinho, R.A. Pancreatic cancer biology and genetics. Nat. Rev. Cancer 2, 897–909 (2002).
Biankin, A.V. et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 491, 399–405 (2012).
Waddell, N. et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518, 495–501 (2015).
Bardeesy, N. et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc. Natl. Acad. Sci. USA 103, 5947–5952 (2006).
Hingorani, S.R. et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4, 437–450 (2003).
Hingorani, S.R. et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7, 469–483 (2005).
Gidekel Friedlander, S.Y. et al. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell 16, 379–389 (2009).
Morton, J.P. et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc. Natl. Acad. Sci. USA 107, 246–251 (2010).
Bardeesy, N. et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 20, 3130–3146 (2006).
Cook, N., Jodrell, D.I. & Tuveson, D.A. Predictive in vivo animal models and translation to clinical trials. Drug Discov. Today 17, 253–260 (2012).
Witkiewicz, A.K. et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 6, 6744 (2015).
Baylin, S.B. & Jones, P.A. A decade of exploring the cancer epigenome—biological and translational implications. Nat. Rev. Cancer 11, 726–734 (2011).
McCleary-Wheeler, A.L. et al. Insights into the epigenetic mechanisms controlling pancreatic carcinogenesis. Cancer Lett. 328, 212–221 (2013).
Delmore, J.E. et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917 (2011).
Filippakopoulos, P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073 (2010).
Lovén, J. et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334 (2013).
Means, A.L. et al. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development 132, 3767–3776 (2005).
Morris, J.P., Cano, D.A., Selkine, S., Wang, S.C. & Hebrok, M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J. Clin. Invest. 120, 508–520 (2010).
Sahai, V. et al. BET bromodomain inhibitors block growth of pancreatic cancer cells in three-dimensional collagen. Mol. Cancer Ther. 13, 1907–1917 (2014).
Von Hoff, D.D. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 369, 1691–1703 (2013).
Sherman, M.H. et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93 (2014).
Ardito, C.M. et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell 22, 304–317 (2012).
Olive, K.P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Singh, M. et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat. Biotechnol. 28, 585–593 (2010).
Garcia, P.L. et al. The BET bromodomain inhibitor JQ1 suppresses growth of pancreatic ductal adenocarcinoma in patient-derived xenograft models. Oncogene doi:10.1038/onc.2015.126 (2015).
Roy, N. et al. Brg1 promotes both tumor-suppressive and oncogenic activities at distinct stages of pancreatic cancer formation. Genes Dev. 29, 658–671 (2015).
Schleger, C., Verbeke, C., Hildenbrand, R., Zentgraf, H. & Bleyl, U. c-MYC activation in primary and metastatic ductal adenocarcinoma of the pancreas: Incidence, mechanisms, and clinical significance. Mod. Pathol. 15, 462–469 (2002).
Lesina, M. et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 19, 456–469 (2011).
Fukuda, A. et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell 19, 441–455 (2011).
McAllister, F. et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 25, 621–637 (2014).
Pylayeva-Gupta, Y., Lee, K.E., Hajdu, C.H., Miller, G. & Bar-Sagi, D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21, 836–847 (2012).
Sodir, N.M. et al. Endogenous Myc maintains the tumor microenvironment. Genes Dev. 25, 907–916 (2011).
Rielland, M. et al. Senescence-associated SIN3B promotes inflammation and pancreatic cancer progression. J. Clin. Invest. 124, 2125–2135 (2014).
Corcoran, R.B. et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 71, 5020–5029 (2011).
Obata, J. et al. p48 subunit of mouse PTF1 binds to RBP-Jkappa/CBF-1, the intracellular mediator of Notch signalling, and is expressed in the neural tube of early stage embryos. Genes Cells 6, 345–360 (2001).
Aguirre, A.J. et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 17, 3112–3126 (2003).
Feldser, D.M. et al. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature 468, 572–575 (2010).
Adhikary, S. et al. Miz1 is required for early embryonic development during gastrulation. Mol. Cell. Biol. 23, 7648–7657 (2003).
Sato, N., Matsubayashi, H., Abe, T., Fukushima, N. & Goggins, M. Epigenetic down-regulation of CDKN1C/p57KIP2 in pancreatic ductal neoplasms identified by gene expression profiling. Clin. Cancer Res. 11, 4681–4688 (2005).
Yan, Y., Frisen, J., Lee, M.H., Massague, J. & Barbacid, M. Ablation of the CDK inhibitor p57(Kip2) results in increased apoptosis and delayed differentiation during mouse development. Genes Dev. 11, 973–983 (1997).
Vlachos, P., Nyman, U., Hajji, N. & Joseph, B. The cell cycle inhibitor p57(Kip2) promotes cell death via the mitochondrial apoptotic pathway. Cell Death Differ. 14, 1497–1507 (2007).
Sánchez-Rivera, F.J. et al. Rapid modelling of cooperating genetic events in cancer through somatic genome editing. Nature 516, 428–431 (2014).
Guerra, C. et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11, 291–302 (2007).
Guerra, C. et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 19, 728–739 (2011).
Zhao, R.Y. et al. CDK inhibitor p57(Kip2) is downregulated by Akt during HER2-mediated tumorigenicity. Cell Cycle 12, 935–943 (2013).
Grasso, C.S. et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat. Med. 21, 555–559 (2015).
De Raedt, T. et al. PRC2 loss amplifies Ras-driven transcription and confers sensitivity to BRD4-based therapies. Nature 514, 247–251 (2014).
Shimamura, T. et al. Efficacy of BET bromodomain inhibition in Kras-mutant non-small cell lung cancer. Clin. Cancer Res. 19, 6183–6192 (2013).
Kawaguchi, Y. et al. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat. Genet. 32, 128–134 (2002).
Jonkers, J. et al. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat. Genet. 29, 418–425 (2001).
de Alboran, I.M. et al. Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity 14, 45–55 (2001).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Mazur, P.K. et al. SMYD3 links lysine methylation of MAP3K2 to Ras-driven cancer. Nature 510, 283–287 (2014).
Matzuk, M.M. et al. Small-molecule inhibition of BRDT for male contraception. Cell 150, 673–684 (2012).
Hruban, R.H. et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 66, 95–106 (2006).
Jameson, K.L. et al. IQGAP1 scaffold-kinase interaction blockade selectively targets RAS-MAP kinase-driven tumors. Nat. Med. 19, 626–630 (2013).
Hsu, P.D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 (2013).
Ran, F.A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Tiscornia, G., Singer, O. & Verma, I.M. Production and purification of lentiviral vectors. Nat. Protoc. 1, 241–245 (2006).
Kim, M.P. et al. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat. Protoc. 4, 1670–1680 (2009).
José, A. et al. Intraductal delivery of adenoviruses targets pancreatic tumors in transgenic Ela-myc mice and orthotopic xenografts. Oncotarget 4, 94–105 (2013).
Qiu, P. et al. Mutation detection using SurveyorTM nuclease. Biotechniques 36, 702–707 (2004).
DuPage, M., Dooley, A.L. & Jacks, T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat. Protoc. 4, 1064–1072 (2009).
Mazur, P.K. et al. Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc. Natl. Acad. Sci. USA 107, 13438–13443 (2010).
Zuber, J. et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature 478, 524–528 (2011).
Debacq-Chainiaux, F., Erusalimsky, J.D., Campisi, J. & Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 4, 1798–1806 (2009).
Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 58, 621–681 (2006).
Chou, T.C. Drug combination sand their synergy quantification using the Chou-Talalay method. Cancer Res. 70, 440–446 (2010).
Carey, M.F., Peterson, C.L. & Smale, S.T. Chromatin immunoprecipitation (ChIP). Cold Spring Harbor Protoc. 10.1101/pdb.prot5279 (2009).
Khatri, P. et al. A common rejection module (CRM) for acute rejection across multiple organs identifies novel therapeutics for organ transplantation. J. Exp. Med. 210, 2205–2221 (2013).
Storey, J.D. A direct approach to false discovery rates. J. R. Stat. Soc. B 64, 479–498 (2002).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Acknowledgements
P.K.M. and A.H. significantly contributed to the work and are listed as co-first authors based on a previous agreement. We thank C. Vakoc (Cold Spring Harbor Laboratory) for sharing shRNA plasmids, M. Winslow (Stanford University) for the R26CAG-tdTomato reporter mice and I. Moreno de Alboran (Spanish National Biotechnology Centre) for MycloxP mice. This work was supported by the German Research Foundation (SFB824/C4 to J.T.S.), the European Union's Seventh Framework Program for research, technological development and demonstration (FP7/CAM-PaC) under grant agreement 602783, the German Cancer Consortium (DKTK) (to R.M.S. and J.T.S.), and the Lustgarten Foundation (J.S.). P.K.M. was supported by the Tobacco-Related Disease Research Program, a Dean's Fellowship from Stanford University, and the Child Health Research Institute and Lucile Packard Foundation for Children's Health at Stanford. J.S. is the Harriet and Mary Zelencik Scientist in Children's Cancer and Blood Diseases. T.K. was supported by a Boehringer Ingelheim Fonds MD Fellowship.
Author information
Authors and Affiliations
Contributions
P.K.M. conceived the study; designed and performed the in vivo studies of pancreatitis-induced PDAC and the Myc knockout studies, and established and performed the five-arm patient-derived PDAC xenograft assays; performed the drug screen, the tissue organoids experiments, the cell culture studies, the ChIP analysis, and the target validation assays in cell lines and in vivo; designed and performed survival treatment of mouse models of PDAC and LAC; designed and performed in vivo CRISPR-Cas9 studies; and coordinated the project, interpreted data, and drafted the manuscript. A.H. performed survival treatment of the Kras;p53 mouse model of PDAC, contributed to MRI animal imaging and tumor volume analysis, contributed to analysis of PDAC xenografts and Myc knockout studies, generated mouse expression arrays, and contributed to cell culture studies and interpretation of the data. S.S.M. and L.D.A. contributed to design and testing of intrapancreatic viral injections. M.W. and G.S. contributed to cell culture studies and interpretation of data. S.H., M.E. and J.K. obtained and prepared surgical tissue samples of PDAC for xenograft and tissue studies. M.T.-A., A.G., R.B., I.H. and P.B.N. performed MRI animal imaging and interpretation and tumor volume analysis. S.M.L. and P.K. performed bioinformatics analyses. S.A.H. and D.V. established and performed treatment of the two-arm PDAC xenograft study and generated human expression arrays. F.J.S.-R. and T.J. designed and constructed pSECC lentivirus. B.S. and I.E., pathologists, performed histological and IHC tissue evaluation and lesion progression quantification. M.H. contributed to IHC analysis. E.H. and V.E. contributed to data interpretation. T.K. contributed to plasmid and lentivirus preparation. L.C.S. and E.A.S.-C. obtained surgical tissue samples of LAC and developed PDX models. J.E.B. contributed to JQ1 inhibitor study protocols and interpretation of data. R.M.S. performed data interpretation. J.S. and J.T.S were equally responsible for supervision of research, data interpretation and manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 (PDF 36873 kb)
Rights and permissions
About this article
Cite this article
Mazur, P., Herner, A., Mello, S. et al. Combined inhibition of BET family proteins and histone deacetylases as a potential epigenetics-based therapy for pancreatic ductal adenocarcinoma. Nat Med 21, 1163–1171 (2015). https://doi.org/10.1038/nm.3952
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3952
This article is cited by
-
Pancreatic cancer acquires resistance to MAPK pathway inhibition by clonal expansion and adaptive DNA hypermethylation
Clinical Epigenetics (2024)
-
Comprehensive review of CRISPR-based gene editing: mechanisms, challenges, and applications in cancer therapy
Molecular Cancer (2024)
-
Epigenetic priming targets tumor heterogeneity to shift transcriptomic phenotype of pancreatic ductal adenocarcinoma towards a Vitamin D susceptible state
Cell Death & Disease (2024)
-
S100PBP is regulated by mutated KRAS and plays a tumour suppressor role in pancreatic cancer
Oncogene (2023)
-
A new vulnerability to BET inhibition due to enhanced autophagy in BRCA2 deficient pancreatic cancer
Cell Death & Disease (2023)