Key Points
-
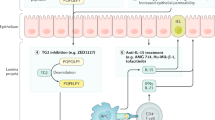
As the knowledge of the pathogenesis of coeliac disease has increased, new alternative treatment modes (such as detoxification of gluten) to replace the burdensome gluten-free diet have been suggested
-
Several investigational approaches exist to detoxify gluten in the lumen of the small intestine, including glutenase therapy and polymeric gluten sequestrants
-
Suggested treatment options that prevent gluten-induced effects at the intestinal epithelium include blockers of intestinal permeability and IL-15
-
Other therapeutic options (such as transglutaminase 2 inhibitors and HLA blockers) are based on the prevention of the gluten-induced immunological cascade in the lamina propria
-
Currently, the most advanced drug candidates are in phase II clinical trials
Abstract
Coeliac disease is a common and fairly well-characterized systemic disorder that mainly affects the small intestine, but also has extraintestinal manifestations. The environmental trigger (gluten derived from wheat, rye and barley), the genetic predisposition conferred by the HLA-DQ2 and HLA-DQ8 haplotypes and many steps in the disease pathogenesis are known. This knowledge has enabled researchers to suggest novel alternative treatments or adjunctive therapies to the gluten-free diet, which is currently the only available and effective treatment for the condition. This Review focuses on emerging and potential treatment strategies that are based on the current concept of the disease pathophysiology. The search for novel future treatment modes, including nonpharmacological and pharmacological approaches, is also outlined. The potential pitfalls associated with the various research avenues are also discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lohiniemi, S. Coeliac disease. Tricky to find, hard to treat, impossible to cure. Lancet 358 (Suppl.), S14 (2001).
Jabri, B. & Sollid, L. M. Tissue-mediated control of immunopathology in coeliac disease. Nat. Rev. Immunol. 9, 858–870 (2009).
Lohi, S. et al. Increasing prevalence of coeliac disease over time. Aliment. Pharmacol. Ther. 26, 1217–1225 (2007).
Kumar, P. J. in Changing Features of Coeliac Disease (eds Lohiniemi, S., Collin, P. & Mäki, M.) 45–49 (The Finnish Coeliac Society, 1998).
Latorre, M. & Green, P. H. The role of corticosteroids in celiac disease. Dig. Dis. Sci. 57, 3039–3041 (2012).
Viljamaa, M. et al. Is coeliac disease screening in risk groups justified? A fourteen-year follow-up with special focus on compliance and quality of life. Aliment. Pharmacol. Ther. 22, 317–324 (2005).
Dewar, D. H. et al. Celiac disease: management of persistent symptoms in patients on a gluten-free diet. World J. Gastroenterol. 18, 1348–1356 (2012).
Shewry, P. R. & Halford, N. G. Cereal seed storage proteins: structures, properties and role in grain utilization. J. Exp. Bot. 53, 947–958 (2002).
van Overbeek, F. M. et al. The daily gluten intake in relatives of patients with coeliac disease compared with that of the general Dutch population. Eur. J. Gastroenterol. Hepatol. 9, 1097–1099 (1997).
Kasarda, D. D. in Coeliac Disease (eds Mäki, M., Collin, P. & Visakorpi, J. K.) 195–212 (Coeliac Disease Study Group, 1997).
Hausch, F., Shan, L., Santiago, N. A., Gray, G. M. & Khosla, C. Intestinal digestive resistance of immunodominant gliadin peptides. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G996–G1003 (2002).
Shan, L. et al. Structural basis for gluten intolerance in celiac sprue. Science 297, 2275–2279 (2002).
Spaenij-Dekking, L. et al. Natural variation in toxicity of wheat: potential for selection of nontoxic varieties for celiac disease patients. Gastroenterology 129, 797–806 (2005).
Di Cagno, R. et al. Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Appl. Environ. Microbiol. 70, 1088–1096 (2004).
Greco, L. et al. Safety for patients with celiac disease of baked goods made of wheat flour hydrolyzed during food processing. Clin. Gastroenterol. Hepatol. 9, 24–29 (2011).
Di Cagno, R. et al. Gluten-free sourdough wheat baked goods appear safe for young celiac patients: a pilot study. J. Pediatr. Gastroenterol. Nutr. 51, 777–783 (2010).
Stoven, S., Murray, J. A. & Marietta, E. Celiac disease: advances in treatment via gluten modification. Clin. Gastroenterol. Hepatol. 10, 859–862 (2012).
Gass, J. & Khosla, C. Prolyl endopeptidases. Cell. Mol. Life Sci. 64, 345–355 (2007).
Garcia-Horsman, J. A. et al. Deficient activity of mammalian prolyl oligopeptidase on the immunoactive peptide digestion in coeliac disease. Scand. J. Gastroenterol. 42, 562–571 (2007).
Mitea, C. et al. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implications for coeliac disease. Gut 57, 25–32 (2008).
Shan, L., Marti, T., Sollid, L. M., Gray, G. M. & Khosla, C. Comparative biochemical analysis of three bacterial prolyl endopeptidases: implications for coeliac sprue. Biochem. J. 383, 311–318 (2004).
Edens, L. et al. Extracellular prolyl endoprotease from Aspergillus niger and its use in the debittering of protein hydrolysates. J. Agric. Food Chem. 53, 7950–7957 (2005).
Marti, T. et al. Prolyl endopeptidase-mediated destruction of T cell epitopes in whole gluten: chemical and immunological characterization. J. Pharmacol. Exp. Ther. 312, 19–26 (2005).
Stepniak, D. et al. Highly efficient gluten degradation with a newly identified prolyl endoprotease: implications for celiac disease. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G621–G629 (2006).
Pyle, G. G. et al. Effect of pretreatment of food gluten with prolyl endopeptidase on gluten-induced malabsorption in celiac sprue. Clin. Gastroenterol. Hepatol. 3, 687–694 (2005).
Bethune, M. T., Strop, P., Tang, Y., Sollid, L. M. & Khosla, C. Heterologous expression, purification, refolding, and structural-functional characterization of EP-B2, a self-activating barley cysteine endoprotease. Chem. Biol. 13, 637–647 (2006).
Gass, J., Vora, H., Bethune, M. T., Gray, G. M. & Khosla, C. Effect of barley endoprotease EP-B2 on gluten digestion in the intact rat. J. Pharmacol. Exp. Ther. 318, 1178–1186 (2006).
Bethune, M. T. et al. A non-human primate model for gluten sensitivity. PLoS ONE 3, e1614 (2008).
Siegel, M. et al. Rational design of combination enzyme therapy for celiac sprue. Chem. Biol. 13, 649–658 (2006).
Gass, J., Bethune, M. T., Siegel, M., Spencer, A. & Khosla, C. Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology 133, 472–480 (2007).
Siegel, M. et al. Safety, tolerability, and activity of ALV003: results from two phase 1 single, escalating-dose clinical trials. Dig. Dis. Sci. 57, 440–450 (2012).
Tye-Din, J. A. et al. The effects of ALV003 pre-digestion of gluten on immune response and symptoms in celiac disease in vivo. Clin. Immunol. 134, 289–295 (2010).
Lähdeaho, M.-L. et al. ALV003, a novel glutanase, attenuates gluten-induced small intestinal mucosal injury in coeliac disease patients: a randomized controlled phase 2a clinical trial. Gut Suppl. 60, A12 (2011).
Stenman, S. et al. Enzymatic detoxification of gluten by germinating wheat proteases: implications for new treatment of celiac disease. Ann. Med. 41, 390–400 (2009).
Stenman, S. et al. Degradation of coeliac disease-inducing rye secalin by germinating cereal enzymes: diminishing toxic effects in intestinal epithelial cells. Clin. Exp. Immunol. 161, 242–249 (2010).
Collado, M. C., Donat, E., Ribes-Koninckx, C., Calabuig, M. & Sanz, Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J. Clin. Pathol. 62, 264–269 (2009).
Wacklin, P. et al. The duodenal microbiota composition of adult celiac disease patients is associated to the clinical manifestation of the disease. Inflamm. Bowel Dis. 19, 934–941 (2013).
Lindfors, K. et al. Live probiotic Bifidobacterium lactis bacteria inhibit the toxic effects induced by wheat gliadin in epithelial cell culture. Clin. Exp. Immunol. 152, 552–558 (2008).
Laparra, J. M. & Sanz, Y. Bifidobacteria inhibit the inflammatory response induced by gliadins in intestinal epithelial cells via modifications of toxic peptide generation during digestion. J. Cell. Biochem. 109, 801–807 (2010).
Laparra, J. M., Olivares, M., Gallina, O. & Sanz, Y. Bifidobacterium longum CECT 7347 modulates immune responses in a gliadin-induced enteropathy animal model. PLoS ONE 7, e30744 (2012).
Smecuol, E. et al. Exploratory, randomized, double-blind, placebo-controlled study on the effects of Bifidobacterium infantis natren life start strain super strain in active celiac disease. J. Clin. Gastroenterol. 47, 139–147 (2013).
De Angelis, M. et al. VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for celiac sprue. Biochim. Biophys. Acta 1762, 80–93 (2006).
Pinier, M. et al. Polymeric binders suppress gliadin-induced toxicity in the intestinal epithelium. Gastroenterology 136, 288–298 (2009).
Pinier, M. et al. The copolymer P(HEMA-co-SS) binds gluten and reduces immune response in gluten-sensitized mice and human tissues. Gastroenterology 142, 316–325 (2012).
Smecuol, E. et al. Gastrointestinal permeability in celiac disease. Gastroenterology 112, 1129–1136 (1997).
Ciccocioppo, R. et al. Altered expression, localization, and phosphorylation of epithelial junctional proteins in celiac disease. Am. J. Clin. Pathol. 125, 502–511 (2006).
Tripathi, A. et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc. Natl Acad. Sci. 106, 16799–16804 (2009).
Lammers, K. M. et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 135, 194–204 e193 (2008).
Di Pierro, M. et al. Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J. Biol. Chem. 276, 19160–19165 (2001).
Clemente, M. G. et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut 52, 218–223 (2003).
Drago, S. et al. Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 41, 408–419 (2006).
Paterson, B. M., Lammers, K. M., Arrieta, M. C., Fasano, A. & Meddings, J. B. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment. Pharmacol. Ther. 26, 757–766 (2007).
Leffler, D. A. et al. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am. J. Gastroenterol. 107, 1554–1562 (2012).
Kelly, C. P. et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment. Pharmacol. Ther. 37, 252–262 (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Rauhavirta, T. et al. Epithelial transport and deamidation of gliadin peptides: a role for coeliac disease patient immunoglobulin A. Clin. Exp. Immunol. 164, 127–136 (2011).
Matysiak-Budnik, T. et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J. Exp. Med. 205, 143–154 (2008).
Mention, J. J. et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology 125, 730–745 (2003).
Maiuri, L. et al. IL-15 drives the specific migration of CD94+ and TCR-γδ+ intraepithelial lymphocytes in organ cultures of treated celiac patients. Am. J. Gastroenterol. 96, 150–156 (2001).
Hüe, S. et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 21, 367–377 (2004).
Ohta, N. et al. IL-15-dependent activation-induced cell death-resistant Th1 type CD8 αβ+NK1.1+ T cells for the development of small intestinal inflammation. J. Immunol. 169, 460–468 (2002).
Maiuri, L. et al. Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology 119, 996–1006 (2000).
Sarra, M. et al. IL-15 positively regulates IL-21 production in celiac disease mucosa. Mucosal Immunol. 6, 244–255 (2013).
Malamut, G. et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J. Clin. Invest. 120, 2131–2143 (2010).
Villanacci, V. et al. Mucosal tissue transglutaminase expression in celiac disease. J. Cell. Mol. Med. 13, 334–340 (2009).
Klock, C. & Khosla, C. Regulation of the activities of the mammalian transglutaminase family of enzymes. Protein Sci. 21, 1781–1791 (2012).
Dieterich, W. et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med. 3, 797–801 (1997).
Molberg, O. et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 4, 713–717 (1998).
Henderson, K. N. et al. A structural and immunological basis for the role of human leukocyte antigen DQ8 in celiac disease. Immunity 27, 23–34 (2007).
Siegel, M. & Khosla, C. Transglutaminase 2 inhibitors and their therapeutic role in disease states. Pharmacol. Ther. 115, 232–245 (2007).
Rauhavirta, T. et al. Are transglutaminase 2 inhibitors able to reduce gliadin-induced toxicity related to celiac disease? A proof-of-concept study. J. Clin. Immunol. 33, 134–142 (2013).
Molberg, O. et al. T cells from celiac disease lesions recognize gliadin epitopes deamidated in situ by endogenous tissue transglutaminase. Eur. J. Immunol. 31, 1317–1323 (2001).
Lebreton, C. et al. Interactions among secretory immunoglobulin A, CD71, and transglutaminase-2 affect permeability of intestinal epithelial cells to gliadin peptides. Gastroenterology 143, 698–707 (2012).
Xia, J. et al. Cyclic and dimeric gluten peptide analogues inhibiting DQ2-mediated antigen presentation in celiac disease. Bioorg. Med. Chem. 15, 6565–6573 (2007).
Kapoerchan, V. V. et al. Design of azidoproline containing gluten peptides to suppress CD4+ T-cell responses associated with celiac disease. Bioorg. Med. Chem. 16, 2053–2062 (2008).
Juse, U., van de Wal, Y., Koning, F., Sollid, L. M. & Fleckenstein, B. Design of new high-affinity peptide ligands for human leukocyte antigen-DQ2 using a positional scanning peptide library. Hum. Immunol. 71, 475–481 (2010).
Huan, J. et al. Single-chain recombinant HLA-DQ2.5/peptide molecules block α2-gliadin-specific pathogenic CD4+ T-cell proliferation and attenuate production of inflammatory cytokines: a potential therapy for celiac disease. Mucosal Immunol. 4, 112–120 (2011).
Sollid, L. M. & Khosla, C. Novel therapies for coeliac disease. J. Intern. Med. 269, 604–613 (2011).
Salvati, V. M. et al. Recombinant human interleukin 10 suppresses gliadin dependent T cell activation in ex vivo cultured coeliac intestinal mucosa. Gut 54, 46–53 (2005).
Mulder, C. J., Wahab, P. J., Meijer, J. W. & Metselaar, E. A pilot study of recombinant human interleukin-10 in adults with refractory coeliac disease. Eur. J. Gastroenterol. Hepatol. 13, 1183–1188 (2001).
Gillett, H. R. et al. Successful infliximab treatment for steroid-refractory celiac disease: a case report. Gastroenterology 122, 800–805 (2002).
Neurath, M. F. & Travis, S. P. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 61, 1619–1635 (2012).
Costantino, G. et al. Treatment of life-threatening type I refractory coeliac disease with long-term infliximab. Dig. Liver Dis. 40, 74–77 (2008).
Reinisch, W. et al. Fontolizumab in moderate to severe Crohn's disease: a phase 2, randomized, double-blind, placebo-controlled, multiple-dose study. Inflamm. Bowel Dis. 16, 233–242 (2010).
Abraham, M. et al. In vitro induction of regulatory T cells by anti-CD3 antibody in humans. J. Autoimmun. 30, 21–28 (2008).
Hmida, N. B. et al. Impaired control of effector T cells by regulatory T cells: a clue to loss of oral tolerance and autoimmunity in celiac disease? Am. J. Gastroenterol. 107, 604–611 (2012).
Edwards, J. C. et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 350, 2572–2581 (2004).
Hauser, S. L. et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 358, 676–688 (2008).
Lindfors, K., Mäki, M. & Kaukinen, K. Transglutaminase 2-targeted autoantibodies in celiac disease: pathogenetic players in addition to diagnostic tools? Autoimmun. Rev. 9, 744–749 (2010).
Mei, H. E. et al. Steady-state generation of mucosal IgA+ plasmablasts is not abrogated by B-cell depletion therapy with rituximab. Blood 116, 5181–5190 (2010).
Simon-Vecsei, Z. et al. A single conformational transglutaminase 2 epitope contributed by three domains is critical for celiac antibody binding and effects. Proc. Natl Acad. Sci. USA 109, 431–436 (2012).
Mora, J. R. & von Andrian, U. H. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 1, 96–109 (2008).
Olaussen, R. W. et al. Reduced chemokine receptor 9 on intraepithelial lymphocytes in celiac disease suggests persistent epithelial activation. Gastroenterology 132, 2371–2382 (2007).
Di Sabatino, A. et al. Increased expression of mucosal addressin cell adhesion molecule 1 in the duodenum of patients with active celiac disease is associated with depletion of integrin α4β7-positive T cells in blood. Hum. Pathol. 40, 699–704 (2009).
Mora, J. R. Homing imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflamm. Bowel Dis. 14, 275–289 (2008).
US National Library of Medicine. ClinicalTrials.gov [online], (2008).
Cassani, B. et al. Gut-tropic T cells that express integrin α4β7 and CCR9 are required for induction of oral immune tolerance in mice. Gastroenterology 141, 2109–2118 (2011).
Wermers, J. D., McNamee, E. N., Wurbel, M. A., Jedlicka, P. & Rivera-Nieves, J. The chemokine receptor CCR9 is required for the T-cell-mediated regulation of chronic ileitis in mice. Gastroenterology 140, 1526–1535 (2011).
Wurbel, M. A., McIntire, M. G., Dwyer, P. & Fiebiger, E. CCL25/CCR9 interactions regulate large intestinal inflammation in a murine model of acute colitis. PLoS ONE 6, e16442 (2011).
Tye-Din, J. A. et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci. Transl. Med. 2, 41ra51 (2010).
Daveson, A. J. et al. Effect of hookworm infection on wheat challenge in celiac disease—a randomised double-blinded placebo controlled trial. PLoS ONE 6, e17366 (2011).
Arentz-Hansen, H. et al. The intestinal T cell response to alpha-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J. Exp. Med. 191, 603–612 (2000).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
Wigren, M. et al. Evidence for a role of regulatory T cells in mediating the atheroprotective effect of apolipoprotein B peptide vaccine. J. Intern. Med. 269, 546–556 (2011).
McSorley, H. J. et al. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS ONE 6, e24092 (2011).
Croese, J., Gaze, S. T. & Loukas, A. Changed gluten immunity in celiac disease by Necator americanus provides new insights into autoimmunity. Int. J. Parasitol. 43, 275–282 (2013).
Acknowledgements
The Celiac Disease Study Group have been financially supported by the Academy of Finland, the Sigrid Juselius Foundation, the Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital (grant numbers 9P020 and 9P033), Elna Kaarina Savolainen's fund allocated for the development of cancer treatment, Pediatric Research Foundation and the European Commission IAPP grant TRANSCOM (Contract number PIA-GA-2010-251506).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
M. Mäki is a member of the Scientific Advisory Board of Alvine Pharmaceuticals, ImmusanT and Flamentera AG and is a member of the Clinical Advisory Board of BioLineRx. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Kaukinen, K., Lindfors, K. & Mäki, M. Advances in the treatment of coeliac disease: an immunopathogenic perspective. Nat Rev Gastroenterol Hepatol 11, 36–44 (2014). https://doi.org/10.1038/nrgastro.2013.141
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2013.141
This article is cited by
-
Celiac Disease Presenting in a Community-Based Gastroenterology Practice: Obesity and Bone Disease Are Common
Digestive Diseases and Sciences (2023)
-
Plant-based and vegetarian diets: an overview and definition of these dietary patterns
European Journal of Nutrition (2023)
-
Mesenchymal Stem Cells to Treat Digestive System Disorders: Progress Made and Future Directions
Current Transplantation Reports (2019)
-
The production of a recombinant tandem single chain fragment variable capable of binding prolamins triggering celiac disease
BMC Biotechnology (2018)
-
Gut Microbiota and Celiac Disease
Digestive Diseases and Sciences (2016)