Abstract
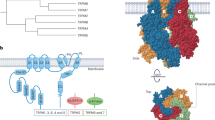
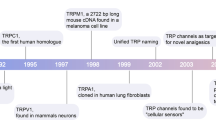
The transient receptor potential (TRP) superfamily consists, in mammals, of six protein subfamilies, TRPC, TRPM, TRPV, TRPA, TRPML and TRPP. TRPs are cation channels involved in many physiological processes and in the pathogenesis of various disorders. In the kidney, TRP channels are expressed along the nephron, and a role for some of these channels in renal function has been proposed. TRPC3 is thought to facilitate calcium ion influx into the principal cells of the collecting duct in response to vasopressin. TRPM3 and TRPV4 might be osmosensors, whereas the TRPP1/TRPP2 complex could function as a mechanosensor in the cilia of renal epithelial cells. A number of kidney diseases have also been linked to dysfunctional activity of TRPs. TRPC6 dysfunction has been associated with the onset of focal segmental glomerosclerosis; TRPP2 dysfunction is linked to autosomal-dominant polycystic kidney disease, TRPM6 mutations underlie hypomagnesemia with secondary hypocalcemia, and TRPV1 dysfunction is implicated in renal hypertension. A link between TRPC1 dysfunction and diabetic nephropathy has also been suggested in an animal model. Animal studies have implicated a role for TRPV5 in idiopathic hypercalciuria and vitamin D-dependent rickets, although these observations have not been confirmed in patients. This Review focuses on the role of renal TRP channels in health and disease.
Key Points
-
Transient receptor potential channels (TRPs) are a family of cation-permeable channels that, depending on the individual protein, are functional as homotetramers or heterotetramers
-
TRPs are also expressed along the nephron, and evidence is now available indicating that malfunction of these channels is involved in hereditary and acquired kidney disorders
-
Reabsorption of Ca2+ and Mg2+ in the distal part of the nephron is facilitated by the epithelial Ca2+ (TRPV5) and Mg2+ (TRPM6) channels
-
Progress in the comprehension of TRP channelopathies will stimulate the development of novel therapeutic strategies
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nilius, B. TRP channels in disease. Biochim. Biophys. Acta 1772, 805–812 (2007).
Hoenderop, J. G. & Bindels, R. J. Calciotropic and magnesiotropic TRP channels. Physiology (Bethesda) 23, 32–40 (2008).
Chang, Q. et al. Molecular determinants in TRPV5 channel assembly. J. Biol. Chem. 279, 54304–54311 (2004).
Clapham, D. E. TRP channels as cellular sensors. Nature 426, 517–524 (2003).
Nilius, B., Owsianik, G., Voets, T. & Peters, J. A. Transient receptor potential cation channels in disease. Physiol. Rev. 87, 165–217 (2007).
Goel, M., Sinkins, W. G., Zuo, C. D., Estacion, M. & Schilling, W. P. Identification and localization of TRPC channels in the rat kidney. Am. J. Physiol. Renal Physiol. 290, F1241–F1252 (2006).
Goel, M., Sinkins, W. G., Zuo, C. D., Hopfer, U. & Schilling, W. P. Vasopressin-induced membrane trafficking of TRPC3 and AQP2 channels in cells of the rat renal collecting duct. Am. J. Physiol. Renal Physiol. 293, F1476–F1488 (2007).
Groenestege, W. M., Hoenderop, J. G., van den Heuvel, L., Knoers, N. & Bindels, R. J. The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. J. Am. Soc. Nephrol. 17, 1035–1043 (2006).
Hanaoka, K. et al. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408, 990–994 (2000).
Wimalawansa, S. J. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr. Rev. 17, 533–585 (1996).
Tian, W. et al. Renal expression of osmotically responsive cation channel TRPV4 is restricted to water-impermeant nephron segments. Am. J. Physiol. Renal Physiol. 287, F17–F24 (2004).
Strotmann, R., Harteneck, C., Nunnenmacher, K., Schultz, G. & Plant, T. D. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2, 695–702 (2000).
Hoenderop, J. G. et al. Localization of the epithelial Ca(2+) channel in rabbit kidney and intestine. J. Am. Soc. Nephrol. 11, 1171–1178 (2000).
Nijenhuis, T., Hoenderop, J. G., van der Kemp, A. W. & Bindels, R. J. Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J. Am. Soc. Nephrol. 14, 2731–2740 (2003).
Lee, N. et al. Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3). J. Biol. Chem. 278, 20890–20897 (2003).
Huang, C. L. The transient receptor potential superfamily of ion channels. J. Am. Soc. Nephrol. 15, 1690–1699 (2004).
Du, J. et al. Canonical transient receptor potential 1 channel is involved in contractile function of glomerular mesangial cells. J. Am. Soc. Nephrol. 18, 1437–1445 (2007).
Worley, P. F. et al. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium 42, 205–211 (2007).
Abramowitz, J. & Birnbaumer, L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 23, 297–328 (2009).
Giunti, S., Barit, D. & Cooper, M. E. Diabetic nephropathy: from mechanisms to rational therapies. Minerva Med. 97, 241–262 (2006).
Jawa, A., Kcomt, J. & Fonseca, V. A. Diabetic nephropathy and retinopathy. Med. Clin. North Am. 88, 1001–1036 (2004).
McGowan, T., McCue, P. & Sharma, K. Diabetic nephropathy. Clin. Lab. Med. 21, 111–146 (2001).
Schäfer, S. et al. Vasopeptidase inhibition prevents nephropathy in Zucker diabetic fatty rats. Cardiovasc. Res. 60, 447–454 (2003).
Phillips, A., Janssen, U. & Floege, J. Progression of diabetic nephropathy. Insights from cell culture studies and animal models. Kidney Blood Press. Res. 22, 81–97 (1999).
Janssen, U., Phillips, A. O. & Floege, J. Rodent models of nephropathy associated with type II diabetes. J. Nephrol. 12, 159–172 (1999).
Niehof, M. & Borlak, J. HNF4α and the Ca-channel TRPC1 are novel disease candidate genes in diabetic nephropathy. Diabetes 57, 1069–1077 (2008).
Winn, M. P. et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 (2005).
Reiser, J. et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 37, 739–744 (2005).
Möller, C. C. et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J. Am. Soc. Nephrol. 18, 29–36 (2007).
Vriens, J. et al. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl Acad. Sci. USA 101, 396–401 (2004).
Mutai, H. & Heller, S. Vertebrate and invertebrate TRPV-like mechanoreceptors. Cell Calcium 33, 471–478 (2003).
Nilius, B., Watanabe, H. & Vriens, J. The TRPV4 channel: structure-function relationship and promiscuous gating behaviour. Pflugers Arch. 446, 298–303 (2003).
Liedtke, W. & Friedman, J. M. Abnormal osmotic regulation in trpv4-/- mice. Proc. Natl Acad. Sci. USA 100, 13698–13703 (2003).
Suzuki, M., Mizuno, A., Kodaira, K. & Imai, M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 278, 22664–22668 (2003).
Mizuno, A., Matsumoto, N., Imai, M. & Suzuki, M. Impaired osmotic sensation in mice lacking TRPV4. Am. J. Physiol. Cell Physiol. 285, C96–C101 (2003).
Leipziger, J. Control of epithelial transport via luminal P2 receptors. Am. J. Physiol. Renal Physiol. 284, F419–F432 (2003).
Gagnon, F., Dulin, N. O., Tremblay, J., Hamet, P. & Orlov, S. N. ATP-induced inhibition of Na+, K+, Cl− cotransport in Madin-Darby canine kidney cells: lack of involvement of known purinoceptor-coupled signaling pathways. J. Membr. Biol. 167, 193–204 (1999).
Silva, G. B. & Garvin, J. L. TRPV4 mediates hypotonicity-induced ATP release by the thick ascending limb. Am. J. Physiol. Renal Physiol. 295, F1090–F1095 (2008).
Köttgen, M. et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J. Cell Biol. 182, 437–447 (2008).
Giamarchi, A. et al. The versatile nature of the calcium-permeable cation channel TRPP2. EMBO Rep. 7, 787–793 (2006).
Chubanov, V. et al. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc. Natl Acad. Sci. USA 101, 2894–2899 (2004).
Schlingmann, K. P. et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 31, 166–170 (2002).
Walder, R. Y. et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat. Genet. 31, 171–174 (2002).
Paunier, L., Radde, I. C., Kooh, S. W., Conen, P. E. & Fraser, D. Primary hypomagnesemia with secondary hypocalcemia in an infant. Pediatrics 41, 385–402 (1968).
Anast, C. S., Mohs, J. M., Kaplan, S. L. & Burns, T. W. Evidence for parathyroid failure in magnesium deficiency. Science 177, 606–608 (1972).
Schlingmann, K. P., Waldegger, S., Konrad, M., Chubanov, V. & Gudermann, T. TRPM6 and TRPM7--Gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta 1772, 813–821 (2007).
Quamme, G. A. Recent developments in intestinal magnesium absorption. Curr. Opin. Gastroenterol. 24, 230–235 (2008).
Chubanov, V. et al. Hypomagnesemia with secondary hypocalcemia due to a missense mutation in the putative pore-forming region of TRPM6. J. Biol. Chem. 282, 7656–7667 (2007).
Topala, C. N. et al. Molecular determinants of permeation through the cation channel TRPM6. Cell Calcium 41, 513–523 (2007).
Li, M., Jiang, J. & Yue, L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 127, 525–537 (2006).
Voets, T. et al. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J. Biol. Chem. 279, 19–25 (2004).
Schmitz, C. et al. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J. Biol. Chem. 280, 37763–37771 (2005).
Groenestege, W. M. et al. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J. Clin. Invest. 117, 2260–2267 (2007).
Naderi, A. S. & Reilly, R. F. Jr. Hereditary etiologies of hypomagnesemia. Nat. Clin. Pract. Nephrol. 4, 80–89 (2008).
Jean, G. W. & Shah, S. R. Epidermal growth factor receptor monoclonal antibodies for the treatment of metastatic colorectal cancer. Pharmacotherapy 28, 742–754 (2008).
Lin, C. C. et al. Phase I study of cetuximab, erlotinib, and bevacizumab in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 63, 1065–1071 (2008).
Guarino, M. J. et al. Dual inhibition of the epidermal growth factor receptor pathway with cetuximab and erlotinib: a phase I study in patients with advanced solid malignancies. Oncologist 14, 119–124 (2009).
Jin, J. et al. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 322, 756–760 (2008).
Ravine, D. et al. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343, 824–827 (1994).
Harris, P. C. & Torres, V. E. Polycystic Kidney Disease. Annu. Rev. Med. 60, 321–337 (2008).
Grantham, J. J. Clinical practice. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 359, 1477–1485 (2008).
Rizk, D. & Chapman, A. Treatment of autosomal dominant polycystic kidney disease (ADPKD): the new horizon for children with ADPKD. Pediatr. Nephrol. 23, 1029–1036 (2008).
Chang, M. Y. & Ong, A. C. Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and treatment. Nephron Physiol. 108, 1–7 (2008).
Harris, P. C. & Torres, V. E. Understanding pathogenic mechanisms in polycystic kidney disease provides clues for therapy. Curr. Opin. Nephrol. Hypertens. 15, 456–463 (2006).
Hateboer, N. et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 353, 103–107 (1999).
Lu, W. et al. Late onset of renal and hepatic cysts in Pkd1-targeted heterozygotes. Nat. Genet. 21, 160–161 (1999).
Lu, W. et al. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat. Genet. 17, 179–181 (1997).
Nauli, S. M. et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129–137 (2003).
Hughes, J. et al. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 10, 151–160 (1995).
Sandford, R. et al. Comparative analysis of the polycystic kidney disease 1 (PKD1) gene reveals an integral membrane glycoprotein with multiple evolutionary conserved domains. Hum. Mol. Genet. 6, 1483–1489 (1997).
Koulen, P. et al. Polycystin-2 is an intracellular calcium release channel. Nat. Cell Biol. 4, 191–197 (2002).
Pak, C. Y., Oata, M., Lawrence, E. C. & Snyder, W. The hypercalciurias. Causes, parathyroid functions, and diagnostic criteria. J. Clin. Invest. 54, 387–400 (1974).
Nijenhuis, T. et al. Thiazide-induced hypocalciuria is accompanied by a decreased expression of Ca2+ transport proteins in kidney. Kidney Int. 64, 555–564 (2003).
van Abel, M. et al. Coordinated control of renal Ca(2+) transport proteins by parathyroid hormone. Kidney Int. 68, 1708–1721 (2005).
Kaplan, R. A., Haussler, M. R., Deftos, L. J., Bone, H. & Pak, C. Y. The role of 1α,25-dihydroxyvitamin D in the mediation of intestinal hyperabsorption of calcium in primary hyperparathyroidism and absorptive hypercalciuria. J. Clin. Invest. 59, 756–760 (1977).
Broadus, A. E., Erickson, S. B., Gertner, J. M., Cooper, K. & Dobbins, J. W. An experimental human model of 1,25-dihydroxyvitamin D-mediated hypercalciuria. J. Clin. Endocrinol. Metab. 59, 202–206 (1984).
Hoenderop, J. G. et al. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J. Clin. Invest. 112, 1906–1914 (2003).
Muller, D. et al. Epithelial Ca(2+) channel (ECAC1) in autosomal dominant idiopathic hypercalciuria. Nephrol. Dial. Transplant. 17, 1614–1620 (2002).
Renkema, K. Y. et al. TRPV5 gene polymorphisms in renal hypercalciuria. Nephrol. Dial. Transplant. 24, 1919–1924 (2009).
Hoenderop, J. G., De Pont, J. J., Bindels, R. J. & Willems, P. H. Hormone-stimulated Ca2+ reabsorption in rabbit kidney cortical collecting system is cAMP-independent and involves a phorbol ester-insensitive PKC isotype. Kidney Int. 55, 225–233 (1999).
Hoenderop, J. G. & Bindels, R. J. Epithelial Ca2+ and Mg2+ channels in health and disease. J. Am. Soc. Nephrol. 16, 15–26 (2005).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Chang, Q. et al. The β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310, 490–493 (2005).
Lu, P., Boros, S., Chang, Q., Bindels, R. J. & Hoenderop, J. G. The β-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6. Nephrol. Dial. Transplant. 23, 3397–3402 (2008).
Bianco, S. D. et al. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J. Bone Miner. Res. 22, 274–285 (2007).
Akey, J. M., Swanson, W. J., Madeoy, J., Eberle, M. & Shriver, M. D. TRPV6 exhibits unusual patterns of polymorphism and divergence in worldwide populations. Hum. Mol. Genet. 15, 2106–2113 (2006).
Suzuki, Y. et al. Gain-of-function haplotype in the epithelial calcium channel TRPV6 is a risk factor for renal calcium stone formation. Hum. Mol. Genet. 17, 1613–1618 (2008).
Dardenne, O., Prudhomme, J., Hacking, S. A., Glorieux, F. H. & St-Arnaud, R. Rescue of the pseudo-vitamin D deficiency rickets phenotype of CYP27B1-deficient mice by treatment with 1,25-dihydroxyvitamin D3: biochemical, histomorphometric, and biomechanical analyses. J. Bone Miner. Res. 18, 637–643 (2003).
Malloy, P. J. & Feldman, D. Vitamin D resistance. Am. J. Med. 106, 355–370 (1999).
Dardenne, O., Prud'homme, J., Arabian, A., Glorieux, F. H. & St-Arnaud, R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1α-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology 142, 3135–3141 (2001).
Panda, D. K., Al Kawas, S., Seldin, M. F., Hendy, G. N. & Goltzman, D. 25-hydroxyvitamin D 1α-hydroxylase: structure of the mouse gene, chromosomal assignment, and developmental expression. J. Bone Miner. Res. 16, 46–56 (2001).
Li, Y. C., Bolt, M. J., Cao, L. P. & Sitrin, M. D. Effects of vitamin D receptor inactivation on the expression of calbindins and calcium metabolism. Am. J. Physiol. Endocrinol. Metab. 281, E558–E564 (2001).
Van Cromphaut, S. J. et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc. Natl Acad. Sci. USA 98, 13324–13329 (2001).
Hoenderop, J. G. et al. Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3-1α-hydroxylase knockout mice. FASEB J. 16, 1398–1406 (2002).
Weber, K., Erben, R. G., Rump, A. & Adamski, J. Gene structure and regulation of the murine epithelial calcium channels ECaC1 and 2. Biochem. Biophys. Res. Commun. 289, 1287–1294 (2001).
Grimm, C., Kraft, R., Sauerbruch, S., Schultz, G. & Harteneck, C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J. Biol. Chem. 278, 21493–21501 (2003).
Oberwinkler, J., Lis, A., Giehl, K. M., Flockerzi, V. & Philipp, S. E. Alternative splicing switches the divalent cation selectivity of TRPM3 channels. J. Biol. Chem. 280, 22540–22548 (2005).
Wagner, T. F. et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat. Cell Biol. 10, 1421–1430 (2008).
Valenti, G., Procino, G., Tamma, G., Carmosino, M. & Svelto, M. Minireview: aquaporin 2 trafficking. Endocrinology 146, 5063–5070 (2005).
Kim, J. Y. et al. Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. J. Biol. Chem. 281, 32540–32549 (2006).
Kopp, U. C., Cicha, M. Z. & Smith, L. A. Dietary sodium loading increases arterial pressure in afferent renal-denervated rats. Hypertension 42, 968–973 (2003).
Wang, Y. & Wang, D. H. A novel mechanism contributing to development of Dahl salt-sensitive hypertension: role of the transient receptor potential vanilloid type 1. Hypertension 47, 609–614 (2006).
Wang, Y., Babánková, D., Huang, J., Swain, G. M. & Wang, D. H. Deletion of transient receptor potential vanilloid type 1 receptors exaggerates renal damage in deoxycorticosterone acetate-salt hypertension. Hypertension 52, 264–270 (2008).
Koomans, H. A., Blankestijn, P. J. & Joles, J. A. Sympathetic hyperactivity in chronic renal failure: a wake-up call. J. Am. Soc. Nephrol. 15, 524–537 (2004).
Acknowledgements
The authors thank Dr J. Schoeber for critically reading the manuscript. This work was financially supported, in part, by grants from the Dutch Kidney Foundation (C03.6017, C06.2170) and the Netherlands Organization for Scientific Research (NWO-ALW 814.02.001, NWO-ALW 816.02.003, NWO-CW 700.55.302, ZonMw 9120.6110). J. G. J. Hoenderop is supported by an EURYI award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Woudenberg-Vrenken, T., Bindels, R. & Hoenderop, J. The role of transient receptor potential channels in kidney disease. Nat Rev Nephrol 5, 441–449 (2009). https://doi.org/10.1038/nrneph.2009.100
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2009.100
This article is cited by
-
Nephrotoxicity evaluation and proteomic analysis in kidneys of rats exposed to thioacetamide
Scientific Reports (2022)
-
Canonical transient receptor potential channels and their modulators: biology, pharmacology and therapeutic potentials
Archives of Pharmacal Research (2021)
-
Elevated FGF23 and disordered renal mineral handling with reduced bone mineralization in chronically erythropoietin over-expressing transgenic mice
Scientific Reports (2019)
-
Transient Receptor Potential Vanilloid 4 Channel Deficiency Aggravates Tubular Damage after Acute Renal Ischaemia Reperfusion
Scientific Reports (2018)
-
Role of renal TRP channels in physiology and pathology
Seminars in Immunopathology (2016)