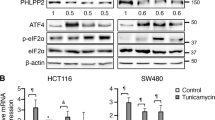
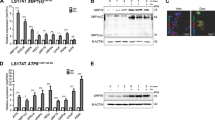
Abstract
The endoplasmic reticulum (ER) stress occurs frequently in cancers. The unfolded protein response (UPR) is activated to cope with ER stress. This has generated widespread interest in targeting UPR as therapeutic strategies. Inositol-requiring transmembrane kinase/endonuclease 1α (IRE1α), an ER stress sensor, is a key component of UPR. However, the role of IRE1α in tumorigenesis remains unclear. The purpose of this work is to investigate the role of IRE1α in colon cancer and to determine whether IRE1α could serve as a target for therapy. We found that knockdown of IRE1α suppressed the proliferation of colon cancer cells in vitro and xenograft growth in vivo. Inhibition of expression of IRE1α decreased stemness of colon cancer stem cells (CSCs) and attenuated growth of intestinal organoids. Genetic ablation of IRE1α prevented the colitis-associated colonic tumorigenesis in mice. The mechanistic study indicates that knockdown of IRE1α repressed the expression of β-catenin, a key factor that drives colonic tumorigenesis, through activating pancreatic ER kinase/eukaryotic translation initiation factor 2α signaling. We found that the IRE1a-specific inhibitor 4μ8C could suppress the production of β-catenin, inhibited the proliferation of colon cancer cells, repressed colon CSCs and prevented xenograft growth. The results suggest that IRE1α has a critical role in colonic tumorigenesis and IRE1α targeting might be a strategy for treatment of colon cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ron D, Walter P . Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007; 8: 519–529.
Todd DJ, Lee AH, Glimcher LH . The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol 2008; 8: 663–674.
Cox JS, Shamu CE, Walter P . Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 1993; 73: 1197–1206.
Morl K, Ma W, Gething MJ, Sambrook J . A transmembrane protein with a cdc2+CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 1993; 74: 743–756.
Hetz C . The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 2012; 13: 89–102.
Sano R, Reed JC . ER stress-induced cell death mechanisms. Biochim Biophys Acta 2013; 1833: 3460–3470.
Wang M, Kaufman RJ . The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer 2014; 14: 581–597.
Oakes SA, Papa FR . The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol 2015; 10: 173–194.
Ma Y, Hendershot LM . The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer 2004; 4: 966–977.
Chevet E, Hetz C, Samali A . Endoplasmic reticulum stress–activated cell reprogramming in oncogenesis. Cancer Discov 2015; 5: 586–597.
Chen X, Iliopoulos D, Zhang Q, Tang Q, Greenblatt MB, Hatziapostolou M et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature 2014; 508: 103–107.
Carrasco DR, Sukhdeo K, Protopopova M, Sinha R, Enos M, Carrasco DE et al. The differentiation and stress response factor XBP1 drives multiple myeloma pathogenesis. Cancer Cell 2007; 11: 349–360.
Gomez BP, Riggins RB, Shajahan AN, Klimach U, Wang A, Crawford AC et al. Human X-Box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. FASEB J 2007; 21: 4013–4027.
Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL et al. ER stress–mediated autophagy promotes Myc-dependent transformation and tumor growth. J Clin Invest 2012; 122: 4621–4634.
Tanjore HD, Cheng S, Degryse AL, Zoz DF, Abdolrasulnia R, Lawson WE et al. Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J Biol Chem 2011; 286: 30972–30980.
Ulianich L, Garbi C, Treglia AS, Punzi D, Miele C, Raciti GA et al. ER stress is associated with dedifferentiation and an epithelial–to -mesenchymal transition-like phenotype in PC Cl3 thyroid cells. J Cell Sci 2008; 121: 477–486.
Feng YX, Sokol ES, Del Vecchio CA, Sanduja S, Claessen JH, Proia TA et al. Epithelial-to-mesenchymal transition activates PERK–eIF2α and sensitizes cells to endoplasmic reticulum stress. Cancer Discov 2014; 4: 702–715.
Clarke HJ, Chambers JE, Liniker ES, Marciniak J . Endoplasmic reticulum stress in malignancy. Cancer Cell 2014; 25: 563–573.
Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G et al. Patterns of somatic mutation in human cancer genomes. Nature 2007; 446: 153–158.
Nagelkerke A, Bussink J, Sweep FC, Span PN . The unfolded protein response as a target for cancer therapy. Biochim Biophys Acta 2014; 1846: 277–284.
Wang M, Kaufman RJ . Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016; 529: 326–335.
Zhao WT, Zhou CF, Li XB, Zhang YF, Fan L, Pelletier J et al. The von Hippel-Lindau protein pVHL inhibits ribosome biogenesis and protein synthesis. J Biol Chem 2013; 288: 16588–16597.
Holland JD, Klaus A, Garratt A, Birchmeier NW . Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol 2013; 25: 254–264.
Vermeulen L, De Sousa E, Melo F, van der Heijden M, Cameron K, de Jong JH et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 2010; 12: 468–476.
Lien WH, Fuchs E . Wnt some lose some: transcriptional governance of stem cells by Wnt/β-catenin signaling. Genes Dev 2014; 28: 1517–1532.
Llado V, Nakanishi Y, Duran A, Reina-Campos M, Shelton PM, Linares JF et al. Repression of intestinal stem cell function and tumorigenesis through direct phosphorylation of β-catenin and Yap by PKCζ. Cell Rep 2015; 10: 740–754.
Anastas JN, Moon RT . WNT signaling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013; 13: 11–26.
Brewer JW, Sherr CJ, Diehl JA . Mammalian unfolded protein response inhibits cyclin D1 translation and cell-cycle progression. Proc Natl Acad Sci USA 1999; 96: 8505–8510.
Brewer JW . PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci USA 2000; 97: 12625–12630.
Zhang F, Hamanaka RB, Bobrovnikova-Marjon E, Gordan JD, Dai MS, Lu H et al. Ribosomal stress couples the unfolded protein response to p53-dependent cell cycle arrest. J Biol Chem 2006; 281: 30036–30045.
Thomas SE, Malzer E, Ordóñez A, EDalton L, van’t Wout EF, Liniker E et al. p53 and translation attenuation regulate distinct cell cycle checkpoints during endoplasmic reticulum (ER) stress. J Biol Chem 2013; 288: 7606–7617.
Klaus A, Birchmeier W . Wnt signalling and its impact on development and cancer. Nat Rev Cancer 2008; 8: 387–398.
Reya T, Clevers H . Wnt signalling in stem cells and cancer. Nature 2005; 434: 843–850.
Heijmans J, van Lidth de Jeude JF, Koo BK, Rosekrans SL, Wielenga MC, van de Wetering V et al. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep 2013; 3: 1128–1139.
Rosekrans SL, Heijmans J, Büller JA, Westerlund J, Lee AS, Muncan V et al. ER stress induces epithelial differentiation in the mouse oesophagus. Gut 2015; 64: 195–202.
Niederreiter L, Fritz MJ, Adolph TE, Krismer AM, Offner FA, Tschurtschenthaler M et al. ER stress transcription factor Xbp1 suppresses intestinal tumorigenesis and directs intestinal stem cells. J Exp Med 2013; 210: 2041–2056.
Mimura N, Fulciniti M, Gorgun G, Tai YT, Cirstea D, Santo L et al. Blockade of XBP1 splicing by inhibition of IRE1α is a promising therapeutic option in multiple myeloma. Blood 2012; 119: 5772–5781.
Jiang D, Niwa M, Koong AC . Targeting the IRE1α–XBP1 branch of the unfolded protein response in human diseases. Semin Cancer Biol 2015; 33: 48–56.
Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood 2011; 117: 1311–1314.
Cross BC, Sadowski PG, Jha BK, Zak J, Goodman JM, Silverman RH et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci USA 2012; 109: E869–E878.
Zhang HS, Chen Y, Fan L, Xi QL, Wu GH, Li XX et al. The endoplasmic reticulum stress sensor IRE1α in Intestinal epithelial cells is essential for protecting against colitis. J Biol Chem 2015; 290: 15327–15336.
Xue J, Li X, Jiao S, Wei Y, Wu G, Fang J et al. Prolyl hydroxylase-3 is down-regulated in colorectal cancer cells and inhibits IKKβ independent of hydroxylase activity. Gastroenterology 2010; 138: 606–615.
Mao T, Shao M, Qiu Y, Huang J, Zhang Y, Song B et al. PKA phosphorylation couples hepatic inositol-requiring enzyme 1alpha to glucagon signaling in glucose metabolism. Proc Natl Acad Sci USA 2011; 108: 15852–15857.
Zhang HS, Yan B, Li XB, Fan L, Zhang YF, Wu GH et al. PAX2 protein induces expression of cyclin D1 through activating AP-1 protein and promotes proliferation of colon cancer cells. J Biol Chem 2012; 287: 44164–44172.
Qiu Y, Mao T, Zhang Y, Shao M, You J, Ding Q et al. A crucial role for RACK1 in the regulation of glucose-stimulated IRE1alpha activation in pancreatic beta cells. Sci Signal 2010; 3: ra7.
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 2009; 459: 262–265.
Sato T, Clevers H . Primary mouse small intestinal epithelial cell cultures. Methods Mol Biol 2013; 945: 319–328.
Acknowledgements
This work was funded by STCSM (16JC1406100), NSFC (31270829, 31470769, 31670785), Chinese Academy of Sciences (XDA08030300, KFJ-EW-STS-099) and CAS/SAFEA International Partnership Program for Creative Research Teams. We thank Dr Xie for kindly providing the vectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Li, XX., Zhang, HS., Xu, YM. et al. Knockdown of IRE1α inhibits colonic tumorigenesis through decreasing β-catenin and IRE1α targeting suppresses colon cancer cells. Oncogene 36, 6738–6746 (2017). https://doi.org/10.1038/onc.2017.284
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.284
This article is cited by
-
Endoplasmic reticulum stress and therapeutic strategies in metabolic, neurodegenerative diseases and cancer
Molecular Medicine (2024)
-
ER Stress Decreases Gene Expression Of Transmembrane Protein 117 Via Activation of PKR-like ER Kinase
Cell Biochemistry and Biophysics (2023)
-
Inhibition of IRE1 RNase activity modulates tumor cell progression and enhances the response to chemotherapy in colorectal cancer
Medical Oncology (2023)
-
Inositol-Requiring Kinase 1 Regulates Apoptosis via Inducing Endoplasmic Reticulum Stress in Colitis Epithelial Cells
Digestive Diseases and Sciences (2021)
-
Calreticulin promotes EMT in pancreatic cancer via mediating Ca2+ dependent acute and chronic endoplasmic reticulum stress
Journal of Experimental & Clinical Cancer Research (2020)