Abstract
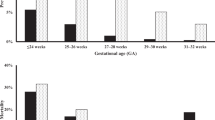
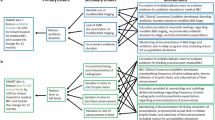
Recent data have revealed declines in the prevalence rates of NEC over the last decade in premature infants. In contrast, SIP has either remained steady or risen during the same epoch. These trends are consistent with our knowledge of the clinical arena. The ability to discern SIP contamination within NEC datasets has slowly improved. Additionally, quality improvement efforts are being utilized to reduce NEC through stewardship of antibiotics, acid inhibitors, central lines and blood products, as well as optimization of human milk diets. These forces are moving us to a new era, where NEC will no longer be the dominant surgical intestinal disease of the extremely preterm neonate. Indeed, in the extremely low birth weight (ELBW) population, SIP may already be the most prevalent reason for abdominal surgery. In this perspective, the reader will find supporting data and references for these assertions as well as predictions for the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Elgendy MM, Othman HF, Heis F, Qattea I, Aly H. Spontaneous intestinal perforation in premature infants: a national study. J Perinatol. 2021;26:455–9.
Gordon PV, Swanson JR, Clark RH. Spontaneous intestinal perforation is not necrotizing enterocolitis but remains the major confounder of NEC data. In: Hackam DJ, editor. Necrotizing enterocolitis, pathogenesis, diagnosis and treatment. London, United Kingdom: CRC Press; 2021.
Fatemizadeh R, Mandal S, Gollins L, Shah S, Premkumar M, Hair A. Incidence of spontaneous intestinal perforations exceeds necrotizing enterocolitis in extremely low birth weight infants fed an exclusive human milk-based diet: a single center experience. J Pediatr Surg. 2020;56:1051–6.
Yang HB, Min JY, Byun J, Ko D, Kim HY, Min KB, et al. Incidence of necrotizing enterocolitis in South Korea from 2007 to 2017. J Perinatol. 2021;41:1092–9.
Javidi D, Wang Z, Rajasekaran S, Hussain N. Temporal and seasonal variations in incidence of stage II and III NEC—a 28 year epidemiologic study from tertiary NICUs in Connecticut, USA. J Perinatol. 2021;41:1100–9.
Blakely ML, Tyson JE, Lally KP, Hintz SR, Eggleston B, Stevenson DK, et al. Initial laparotomy versus peritoneal drainage in extremely low birthweight infants with surgical necrotizing enterocolitis or isolated intestinal perforation: a multicenter randomized clinical trial. Ann Surg. 2021;274:e370–80.
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–51.
Karila K, Anttila A, Iber T, Pakarinen M, Koivusalo A. Outcomes of surgery for necrotizing enterocolitis and spontaneous intestinal perforation in Finland during 1986-2014. J Pediatr Surg. 2018;53:1928–32.
Wadhawan R, Oh W, Hintz SR, Blakely ML, Das A, Bell EF,NICHD Neonatal Research Network, et al. Neurodevelopmental outcomes of extremely low birth weight infants with spontaneous intestinal perforation or surgical necrotizing enterocolitis. J Perinatol. 2014;34:64–70.
Maffei D, Schanler RJ. Human milk is the feeding strategy to prevent necrotizing enterocolitis! Semin Perinatol. 2017;41:36–40.
Patel AL, Kim JH. Human milk and necrotizing enterocolitis. Semin Pediatr Surg. 2018;27:34–8.
Manthe ED, Perks PH, Swanson JR. Team-based implementation of an exclusive human milk diet. Adv Neonatal Care. 2019;19:460–7.
de Lange IH, van Gorp C, Eeftinck Schattenkerk LD, van Gemert WG, Derikx JPM, Wolfs TGAM. Enteral feeding interventions in the prevention of necrotizing enterocolitis: a systematic review of experimental and clinical studies. Nutrients. 2021;13:1726.
Gephart SM, Newnam K, Wyles C, Bethel C, Porter C, Quinn MC, et al. Development of the NEC-Zero toolkit: supporting reliable implementation of necrotizing enterocolitis prevention and recognition. Neonatal Netw. 2020;39:6–15.
Jasani B, Patole S. Standardized feeding regimen for reducing necrotizing enterocolitis in preterm infants: an updated systematic review. J Perinatol. 2017;37:827–33.
Ting JY, Roberts A, Sherlock R, Ojah C, Cieslak Z, Dunn M,Canadian Neonatal Network Investigators, et al. Duration of initial empirical antibiotic therapy and outcomes in very low birth weight infants. Pediatrics. 2019;143:e20182286.
Cantey JB, Pyle AK, Wozniak PS, Hynan LS, Sanchez PJ. Early antibiotic exposure and adverse outcomes in preterm, very low birth weight infants. J Pediatr. 2018;203:62–7.
More K, Athalye-Jape G, Rao S, Patole S. Association of inhibitors of gastric acid secretion and higher incidence of necrotizing enterocolitis in preterm very low-birth-weight infants. Am J Perinatol. 2013;30:849–56.
Guillet R, Stoll BJ, Cotton CM, Gantz M, McDonald S, Poole WK, et al. National Institute of Child Health and Human Development Neonatal Research Network. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006;117:e137–42.
Barr PA, Mally PV, Caprio MC. Standardized nutrition protocol for very low-birth-weight infants resulted in less use of parenteral nutrition and associated complications, better growth, and lower rates of necrotizing enterocolitis. JPEN J Parenter Enter Nutr. 2019;43:540–9.
Chu S, Procaskey A, Tripp S, Naples M, White H, Rhein L. Quality improvement initiative to decrease time to full feeds and central line utilization among infants born less than or equal to 32 0/7 weeks through compliance with standardized feeding guidelines. J Perinatol. 2019;39:1140–8.
Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA. 2016;315:889–97.
Jasani B, Rao S, Patole S. Withholding feeds and transfusion-associated necrotizing enterocolitis in preterm infants: a systematic review. Adv Nutr. 2017;8:764–9.
Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7.
Kahn DJ, Gregorisch S, Whitehouse JS, Fisher PD. Delayed diagnosis of spontaneous intestinal perforation among very low birth weight neonates: a single center experience. J Perinatol. 2019;39:1509–20.
Swanson JR. Surgical necrotizing enterocolitis: time for a definition. J Am Coll Surg. 2015;220:370.
Gordon PV, Swanson JR, MacQueen BC, Christensen RD. A critical question for NEC researchers: can we create a consensus definition of NEC that facilitates research progress? Semin Perinatol. 2017;41:7–14.
Poindexter B. Committee on fetus and newborn. Use of probiotics in preterm infants. Pediatrics. 2021;147:e2021051485.
Herman AC, Carlisle EM, Paxton JB, Gordon PV. Insulin-like growth factor-I governs submucosal growth and thickness in the newborn mouse ileum. Pediatr Res. 2004;55:507–13.
Gordon PV, Young ML, Marshall DD. Focal small bowel perforation: an adverse effect of early postnatal dexamethasone therapy in extremely low birth weight infants. J Perinatol. 2001;21:156–60.
Gordon PV, Moats-Staats BM, Stiles AD, Price WA. Dexamethasone changes the composition of insulin-like growth factor binding proteins in the newborn mouse ileum. J Pediatr Gastroenterol Nutr. 2002;35:532–8.
Gordon PV, Attridge JT. Understanding clinical literature relevant to spontaneous intestinal perforations. Am J Perinatol. 2009;26:309–16.
Gordon P, Rutledge J, Sawin R, Thomas S, Woodrum D. Early postnatal dexamethasone increases the risk of focal small bowel perforation in extremely low birth weight infants. J Perinatol. 1999;19:573–7.
Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, et al. Adverse effects of early dexamethasone treatment in extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 2001;344:95–101.
Attridge JT, Clark R, Walker MW, Gordon PV. New insights into spontaneous intestinal perforation using a national data set: (1) SIP is associated with early indomethacin exposure. J Perinatol. 2006;26:93–9.
Gordon PV, Swanson JR, Clark R. Antenatal indomethacin is more likely associated with spontaneous intestinal perforation rather than NEC. Am J Obstet Gynecol. 2008;198:725.
Stavel M, Wong J, Cieslak Z, Sherlock R, Claveau M, Shah PS. Effect of prophylactic indomethacin administration and early feeding on spontaneous intestinal perforation in extremely low-birth-weight infants. J Perinat. 2017;37:188–93.
Kandraju H, Kanungo J, Lee KS, Daspal S, Adie MA, Dorling J, et al. Canadian Neonatal Network (CNN); Canadian Preterm Birth Network (CPTBN) Investigators. Association of co-exposure of antenatal steroid and prophylactic indomethacin with spontaneous intestinal perforation. J Pediatr. 2021;S0022-3476:00226–2.
Paquette L, Friedlich P, Ramanathan R, Seri I. Concurrent use of indomethacin and dexamethasone increases the risk of spontaneous intestinal perforation in very low birth weight neonates. J Perinatol. 2006;26:486–92.
Gordon PV. Understanding intestinal vulnerability to perforation in the extremely low birth weight infant. Pediatr Res. 2009;65:138–44.
Gordon PV, Paxton JB, Fox NS. The cellular repressor of E1A-stimulated genes mediates glucocorticoid-induced lows of the type-2 IGF-receptor in ileal epithelial cells. J Endocrinol. 2005;185:265–73.
Gordon PV, Paxton JB, Kuemmerle JF, Fox NS. A 14-dDa cathepsin L-derived carboxyl IGFBP-2 fragment is sequestered by cultured rat ileal crypt cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G79–87.
Gordon PV, Herman AC, Marcinkiewicz M, Gaston BM, Laubach VE, Aschner JL. A neonatal mouse model of intestinal perforation: investigating the harmful synergism between glucocorticoids and indomethacin. J Pediatr Gastroenterol Nutr. 2007;45:509–19.
Battersby C, Longford N, Costeloe K, Modi N,UK Neonatal Collaborative Necrotising Enterocolitis Study Group. Development of a gestational age–specific case definition for neonatal necrotizing enterocolitis. JAMA Pediatr. 2017;171:256–63.
Gephart SM, Gordon PV, Penn AH, Gregory KE, Swanson JR, Maheshwari A, et al. Changing the paradigm of defining, detecting, and diagnosing NEC: perspectives on Bell’s stages and biomarkers for NEC. Semin Pediatr Surg. 2018;27:3–10.
Ji J, Ling XB, Zhao Y, Hu Z, Zheng X, Xu Z, et al. A data-driven algorithm integrating clinical and laboratory features for the diagnosis and prognosis of necrotizing enterocolitis. PLoS ONE. 2014;9:e89860.
Caplan MS, Underwood MA, Modi N, Patel R, Gordon PV, Sylvester KG, et al. Necrotizing enterocolitis: using regulatory science and drug development to improve outcomes. J Pediatr. 2019;212:208–15.
Centers for Disease Control. CDC/NHSN Surveillance definitions for specific types of infections. 2021. https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf.
Vermont Oxford Network. 2021 Manual of Operations, Part 2, Release 25.0. 2021. https://vtoxford.zendesk.com/hc/en-us/articles/360056768093-2021-Manual-of-Operations-Part-2-Release-25-0-PDF.
Lueschow SR, Boly TJ, Jasper E, Patel RM, McElroy SJ. A critical evaluation of current definitions of necrotizing enterocolitis. Pediatr Res. 2021. https://doi.org/10.1038/s41390-021-01570-y. Online ahead of print.
Gordon PV, Clark R, Swanson JR, Spitzer A. Can a national dataset generate a nomogram for necrotizing enterocolitis onset? J Perinatol. 2014;34:732–5.
Challis P, Larsson L, Sjostrom ES, Serenius F, Domellof M, Elfvin A. Validation of the diagnosis of necrotising enterocolitis in a Swedish population-based observational study. Acta Paediatr. 2019;108:835–41.
Moss RL, Dimmitt RA, Barnhart DC, Sylvester KG, Brown RL, Powell DM, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med. 2006;354:2225–34.
Rees CM, Eaton S, Kiely EM, Wade AM, McHugh K, Pierro A. Peritoneal drainage or laparotomy for neonatal bowel perforation? A randomized controlled trial. Ann Surg. 2008;248:44–51.
Stewart CJ, Estes MK, Ramani S. Establishing human intestinal enteroid/organoid lines from preterm infant and adult tissue. Methods Mol Biol. 2020;2121:185–98.
De Fazio L, Beghetti I, Bertuccio SN, Marsico C, Martini S, Masetti R, et al. Necrotizing enterocolitis: overview on in vitro models. Int J Mol Sci. 2021;22:6761.
Kovler ML, Sodhi CP, Hackam DJ. Precision-based modeling approaches for necrotizing enterocolitis. Dis Model Mech. 2020;13:dmm044388.
Chan KY, Leung KT, Tam YH, Lam HS, Cheung HM, Ma TPY, et al. Genome-wide expression profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in human intestinal tissues. Ann Surg. 2014;260:1128–37.
Chan KY, Leung FL, Lam HS, Tam YH, To KF, Cheung HM, et al. Immunoregulatory protein profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in preterm infants. PLoS ONE. 2012;7:e36977.
Ng PC, Chan KY, Leung KT, Tam YH, Ma TY, Lam HS, et al. Comparative MiRNA expressional profiles and molecular networks in human small bowel tissues of necrotizing enterocolitis and spontaneous intestinal perforation. PLoS ONE. 2015;10:e0135737.
International Classification of Diseases and Related health Problems, 10th revision. 2021. https://icd.who.int/browse10/2019/en.
ICD-11 for Mortality and Morbidity Statistics (ICD-11 MMS). 2021. https://icd.who.int/browse11/l-m/en.
Gordon PV, Swanson JR, Attridge JT, Clark R. Emerging trends in acquired neonatal intestinal disease: is it time to abandon Bell’s criteria? J Perinatol. 2007;27:661–71.
Jasani B, Rao S, Patole S. Withholding feeds and transfusion-associated necrotizing enterocolitis in preterm infants: a systematic review. Adv Nutr. 2017;8:764–9.
Maheshwari A, Patel RM, Christensen RD. Anemia, red blood cell transfusions, and necrotizing enterocolitis. Semin Pediatr Surg. 2018;27:47–51.
Cordova J, Sriram S, Patton T, Jericho H, Gokhale R, Weinstein D, et al. Manifestations of cow’s milk protein intolerance in preterm infants. J Pediatr Gastroenterol Nutr. 2016;62:140–4.
Lenfestey MW, de la Cruz D, Neu J. Food protein-induced enterocolitis instead of necrotizing enterocolitis? A neonatal intensive care unit case series. J Pediatr. 2018;200:270–3.
Le Kim Y, Joo JY, Jung YH, Choi CW, Kim BI, Yang HR. Differentiation of food protein-induced enterocolitis syndrome misleading to necrotizing enterocolitis. Ann Allergy Asthma Immunol. 2021;S1081-1206:01124–8.
Christensen RD, Lambert DK, Baer VL, Gordon PV. Necrotizing enterocolitis in term infants. Clin Perinatol. 2013;40:69–78.
Lambert DK, Christensen RD, Henry E, Besner GE, Baer VL, Wiedmeier SE, et al. Necrotizing enterocolitis in term neonates: data from a multihospital health-care system. J Perinatol. 2007;27:437–43.
Gordon PV. The little database that could: Intermountain Health Care and the uphill quest for prevention of term necrotizing enterocolitis. J Perinatol. 2007;27:397–8.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the manuscript. RHC contributed to data acquisition and all authors contributed to data analysis. JRS and PVG drafted the original manuscript. AH and RHC reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable to all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
JRS is an associate editor for the Journal of Perinatology and has received speaking honoraria from Prolacta Bioscience, Inc. AH receives research support from Prolacta Bioscience for the investigator-initiated randomized cream trial. PVG and RHC have no conflicts to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Swanson, J.R., Hair, A., Clark, R.H. et al. Spontaneous intestinal perforation (SIP) will soon become the most common form of surgical bowel disease in the extremely low birth weight (ELBW) infant. J Perinatol 42, 423–429 (2022). https://doi.org/10.1038/s41372-022-01347-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01347-z
This article is cited by
-
Risk factors and epidemiology of spontaneous intestinal perforation among infants born at 22–24 weeks’ gestational age
Journal of Perinatology (2024)
-
Comparison of preoperative and intraoperative surgeon diagnosis and pathologic findings in spontaneous intestinal perforation vs necrotizing enterocolitis
Journal of Perinatology (2024)
-
Outcomes by disease onset, sex, and intervention in neonates with SIP and surgical NEC
Pediatric Research (2024)
-
Wide use of broad-spectrum antibiotics in very low birth weight infants with spontaneous focal intestinal perforation—is it really justified?
Infection (2024)
-
Effects of prophylactic probiotics supplementation on infants born very preterm or very low birth weight
Journal of Perinatology (2023)