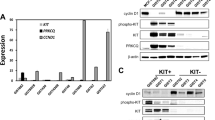
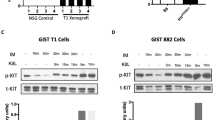
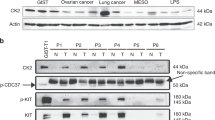
Abstract
KIT/PDGFRA oncogenic tyrosine kinase signaling is the central oncogenic event in most gastrointestinal stromal tumors (GIST), which are human malignant mesenchymal neoplasms that often feature myogenic differentiation. Although targeted inhibition of KIT/PDGFRA provides substantial clinical benefit, GIST cells adapt to KIT/PDGFRA driver suppression and eventually develop resistance. The specific molecular events leading to adaptive resistance in GIST remain unclear. By using clinically representative in vitro and in vivo GIST models and GIST patients’ samples, we found that the E3 ubiquitin ligase Atrogin-1 (FBXO32)—the main effector of muscular atrophy in cachexia—resulted in the most critical gene derepressed in response to KIT inhibition, regardless the type of KIT primary or secondary mutation. Atrogin-1 in GISTs is transcriptionally controlled by the KIT-FOXO3a axis, thus indicating overlap with Atrogin-1 regulation mechanisms in nonneoplastic muscle cells. Further, Atrogin-1 overexpression was a GIST-cell-specific pro-survival mechanism that enabled the adaptation to KIT-targeted inhibition by apoptosis evasion through cell quiescence. Buttressed on these findings, we established in vitro and in vivo the preclinical proof-of-concept for co-targeting KIT and the ubiquitin pathway to maximize the therapeutic response to first-line imatinib treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Cancer Netw. 2010;8:S1–41.
Serrano C, George S. Gastrointestinal stromal tumor: challenges and opportunities for a new decade. Clin Cancer Res. 2020;26:5078–85. https://doi.org/10.1158/1078-0432.CCR-20-1706
Taylor BS, Barretina J, Maki RG, Antonescu CR, Singer S, Ladanyi M. Advances in sarcoma genomics and new therapeutic targets. Nat Rev Cancer. 2011;11:541–57. https://doi.org/10.1038/nrc3087
Wang Y, Marino-Enriquez A, Bennett RR, Zhu M, Shen Y, Eilers G, et al. Dystrophin is a tumor suppressor in human cancers with myogenic programs. Nat Genet. 2014;46:601–6. https://doi.org/10.1038/ng.2974
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80.
Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. https://doi.org/10.1126/science.1079666
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl J Med. 2002;347:472–80. https://doi.org/10.1056/NEJMoa020461
Liegl B, Kepten I, Le C, Zhu M, Demetri GD, Heinrich MC, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol. 2008;216:64–74. https://doi.org/10.1002/path.2382
Serrano C, Marino-Enriquez A, Tao DL, Ketzer J, Eilers G, Zhu M, et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br J cancer. 2019;120:612–20. https://doi.org/10.1038/s41416-019-0389-6
Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24:4764–74. https://doi.org/10.1200/JCO.2006.06.2265
Liu Y, Perdreau SA, Chatterjee P, Wang L, Kuan SF, Duensing A. Imatinib mesylate induces quiescence in gastrointestinal stromal tumor cells through the CDH1-SKP2-p27Kip1 signaling axis. Cancer Res. 2008;68:9015–23. https://doi.org/10.1158/0008-5472.CAN-08-1935
Boichuk S, Parry JA, Makielski KR, Litovchick L, Baron JL, Zewe JP, et al. The DREAM complex mediates GIST cell quiescence and is a novel therapeutic target to enhance imatinib-induced apoptosis. Cancer Res. 2013;73:5120–9. https://doi.org/10.1158/0008-5472.CAN-13-0579
Cohen NA, Zeng S, Seifert AM, Kim TS, Sorenson EC, Greer JB, et al. Pharmacological inhibition of KIT activates MET signaling in gastrointestinal stromal tumors. Cancer Res. 2015;75:2061–70. https://doi.org/10.1158/0008-5472.CAN-14-2564
Li F, Huynh H, Li X, Ruddy DA, Wang Y, Ong R, et al. FGFR-mediated reactivation of MAPK signaling attenuates antitumor effects of imatinib in gastrointestinal stromal tumors. Cancer Discov. 2015;5:438–51. https://doi.org/10.1158/2159-8290.CD-14-0763
Duensing A, Medeiros F, McConarty B, Joseph NE, Panigrahy D, Singer S, et al. Mechanisms of oncogenic KIT signal transduction in primary gastrointestinal stromal tumors (GISTs). Oncogene. 2004;23:3999–4006. https://doi.org/10.1038/sj.onc.1207525
Bauer S, Duensing A, Demetri GD, Fletcher JA. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene. 2007;26:7560–8. https://doi.org/10.1038/sj.onc.1210558
Zhu MJ, Ou WB, Fletcher CD, Cohen PS, Demetri GD, Fletcher JA. KIT oncoprotein interactions in gastrointestinal stromal tumors: therapeutic relevance. Oncogene. 2007;26:6386–95. https://doi.org/10.1038/sj.onc.1210464
Chi P, Chen Y, Zhang L, Guo X, Wongvipat J, Shamu T, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature. 2010;467:849–53. https://doi.org/10.1038/nature09409
Bosbach B, Rossi F, Yozgat Y, Loo J, Zhang JQ, Berrozpe G, et al. Direct engagement of the PI3K pathway by mutant KIT dominates oncogenic signaling in gastrointestinal stromal tumor. Proc Natl Acad Sci USA. 2017;114:E8448–E57. https://doi.org/10.1073/pnas.1711449114
Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98:14440–5. https://doi.org/10.1073/pnas.251541198
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. https://doi.org/10.1016/s0092-8674(04)00400-3
Bardia A, Gounder M, Rodon J, Janku F, Lolkema MP, Stephenson JJ, et al. Phase Ib study of combination therapy with MEK inhibitor binimetinib and phosphatidylinositol 3-kinase inhibitor buparlisib in patients with advanced solid tumors with RAS/RAF alterations. Oncologist. 2020;25:e160–e9. https://doi.org/10.1634/theoncologist.2019-0297
Frolov A, Chahwan S, Ochs M, Arnoletti JP, Pan ZZ, Favorova O, et al. Response markers and the molecular mechanisms of action of Gleevec in gastrointestinal stromal tumors. Mol Cancer Ther. 2003;2:699–709.
Chou JL, Su HY, Chen LY, Liao YP, Hartman-Frey C, Lai YH, et al. Promoter hypermethylation of FBXO32, a novel TGF-beta/SMAD4 target gene and tumor suppressor, is associated with poor prognosis in human ovarian cancer. Lab Investig. 2010;90:414–25. https://doi.org/10.1038/labinvest.2009.138
Ciarapica R, De Salvo M, Carcarino E, Bracaglia G, Adesso L, Leoncini PP, et al. The Polycomb group (PcG) protein EZH2 supports the survival of PAX3-FOXO1 alveolar rhabdomyosarcoma by repressing FBXO32 (Atrogin1/MAFbx). Oncogene. 2014;33:4173–84. https://doi.org/10.1038/onc.2013.471
Zhou H, Liu Y, Zhu R, Ding F, Wan Y, Li Y, et al. FBXO32 suppresses breast cancer tumorigenesis through targeting KLF4 to proteasomal degradation. Oncogene. 2017;36:3312–21. https://doi.org/10.1038/onc.2016.479
van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol cell Biol. 2007;8:440–50. https://doi.org/10.1038/nrm2190
Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–48. https://doi.org/10.1038/ncb1676
Tenbaum SP, Ordonez-Moran P, Puig I, Chicote I, Arques O, Landolfi S, et al. beta-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med. 2012;18:892–901. https://doi.org/10.1038/nm.2772
Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, et al. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem. 2005;280:20814–23. https://doi.org/10.1074/jbc.M500528200
Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–51. https://doi.org/10.1038/nrm1471
Wang D, Zhang Q, Blanke CD, Demetri GD, Heinrich MC, Watson JC, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol. 2012;19:1074–80. https://doi.org/10.1245/s10434-011-2190-5
Hyer ML, Milhollen MA, Ciavarri J, Fleming P, Traore T, Sappal D, et al. A small-molecule inhibitor of the ubiquitin activating enzyme for cancer treatment. Nat Med. 2018;24:186–93. https://doi.org/10.1038/nm.4474
Jin J, Li X, Gygi SP, Harper JW. Dual E1 activation systems for ubiquitin differentially regulate E2 enzyme charging. Nature. 2007;447:1135–8. https://doi.org/10.1038/nature05902
Hemming ML, Lawlor MA, Zeid R, Lesluyes T, Fletcher JA, Raut CP, et al. Gastrointestinal stromal tumor enhancers support a transcription factor network predictive of clinical outcome. Proc Natl Acad Sci USA. 2018;115:E5746–E55. https://doi.org/10.1073/pnas.1802079115
Grunewald S, Klug LR, Muhlenberg T, Lategahn J, Falkenhorst J, Town A, et al. Resistance to avapritinib in PDGFRA-driven GIST is caused by secondary mutations in the PDGFRA kinase domain. Cancer Discov. 2020. https://doi.org/10.1158/2159-8290.CD-20-0487
Serrano C, Leal A, Kuang Y, Morgan JA, Barysauskas CM, Phallen J, et al. Phase I study of rapid alternation of sunitinib and regorafenib for the treatment of tyrosine kinase inhibitor refractory gastrointestinal stromal tumors. Clin Cancer Res. 2019;25:7287–93. https://doi.org/10.1158/1078-0432.CCR-19-2150
Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26:5352–9. https://doi.org/10.1200/JCO.2007.15.7461
Dolly SO, Wagner AJ, Bendell JC, Kindler HL, Krug LM, Seiwert TY, et al. Phase I study of apitolisib (GDC-0980), dual phosphatidylinositol-3-kinase and mammalian target of rapamycin kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2016;22:2874–84. https://doi.org/10.1158/1078-0432.CCR-15-2225
Ran L, Chen Y, Sher J, Wong EWP, Murphy D, Zhang JQ, et al. FOXF1 defines the core-regulatory circuitry in gastrointestinal stromal tumor. Cancer Discov. 2018;8:234–51. https://doi.org/10.1158/2159-8290.CD-17-0468
Tintignac LA, Lagirand J, Batonnet S, Sirri V, Leibovitch MP, Leibovitch SA. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem. 2005;280:2847–56. https://doi.org/10.1074/jbc.M411346200
Lagirand-Cantaloube J, Offner N, Csibi A, Leibovitch MP, Batonnet-Pichon S, Tintignac LA, et al. The initiation factor eIF3-f is a major target for atrogin1/MAFbx function in skeletal muscle atrophy. EMBO J. 2008;27:1266–76. https://doi.org/10.1038/emboj.2008.52
Gupta A, Roy S, Lazar AJ, Wang WL, McAuliffe JC, Reynoso D, et al. Autophagy inhibition and antimalarials promote cell death in gastrointestinal stromal tumor (GIST). Proc Natl Acad Sci USA. 2010;107:14333–8. https://doi.org/10.1073/pnas.1000248107
Garcia-Valverde A, Rosell J, Serna G, Valverde C, Carles J, Nuciforo P, et al. Preclinical activity of PI3K inhibitor copanlisib in gastrointestinal stromal tumor. Mol Cancer Ther. 2020;19:1289–97. https://doi.org/10.1158/1535-7163.MCT-19-1069
Garner AP, Gozgit JM, Anjum R, Vodala S, Schrock A, Zhou T, et al. Ponatinib inhibits polyclonal drug-resistant KIT oncoproteins and shows therapeutic potential in heavily pretreated gastrointestinal stromal tumor (GIST) patients. Clin Cancer Res. 2014;20:5745–55. https://doi.org/10.1158/1078-0432.CCR-14-1397
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. https://doi.org/10.1038/nmeth.2019
Maurel J, Lopez-Pousa A, Calabuig S, Bague S, Del Muro XG, Sanjuan X, et al. Phosphorylated-insulin growth factor I receptor (p-IGF1R) and metalloproteinase-3 (MMP3) expression in advanced gastrointestinal stromal tumors (GIST). A GEIS 19 study. Clin Sarcoma Res. 2016;6:10 https://doi.org/10.1186/s13569-016-0050-6
Pascual-Reguant L, Blanco E, Galan S, Le Dily F, Cuartero Y, Serra-Bardenys G, et al. Lamin B1 mapping reveals the existence of dynamic and functional euchromatin lamin B1 domains. Nat Commun. 2018;9:3420 https://doi.org/10.1038/s41467-018-05912-z
Vitiello GA, Bowler TG, Liu M, Medina BD, Zhang JQ, Param NJ, et al. Differential immune profiles distinguish the mutational subtypes of gastrointestinal stromal tumor. J Clin Investig. 2019;129:1863–77. https://doi.org/10.1172/JCI124108
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47 https://doi.org/10.1093/nar/gkv007
Acknowledgements
This project was funded by the 2014 SARC International Career Development Award (SARC Sarcoma Spore 1U54CA168512–01), Fundación Mari Paz Jiménez Casado, FERO Foundation, Spanish Society of Medical Oncology (SEOM), PERIS SLT006/17/221, ISCIII PI16/01371 and PI19/01271, all to C.S. ISCIII FI20/00275 (to DG-P), and a Ph.D. fellowship from the National Secretary for Higher Education, Science, Technology and Innovation of Ecuador (SENESCYT) (to DFP-J). AE-C is funded by ISCIII PT17/0009/0019 and co-funded by FEDER. We thank the Cellex Foundation for providing facilities and equipment.
Author information
Authors and Affiliations
Contributions
All authors contributed to this study. Conception and design: AG-V, GDD, JAF, JA, and CS. Data acquisition: AG-V, JR, DFP-J, IO-R, EA, JM, JR-C, AE, MG, and CS. Data analysis: AG-V, JR, SS, DGP, EA, AE, MG, and CS. Data interpretation: AG-V, JR, SS, DGP, CV, JC, JAF, JA, and CSG. Writing: AG-V and CS. All authors revisited and approved the draft.
Corresponding author
Ethics declarations
Competing interests
SS has received consulting fees (advisory role) from Boehringer Ingelheim. DFP-J has received travel grants from Pfizer, Roemmers, and Roche. MG has received consulting fees from Roche and Illumina. CV has received consulting fees (advisory role) from Lilly, PharmaMar, Pfizer, Eisai, Bayer, Mundipharma, GlaxoSmithKline, and travel grants from PharmaMar, Lilly, Novartis, Bayer, and Pfizer. CS has received research funding (institution) from Karyopharm, Pfizer, Inc, Deciphera Pharmaceuticals, and Bayer AG; consulting fees (advisory role) from CogentBio, Immunicum AB, Deciphera Pharmaceuticals and Blueprint Medicines; payment for lectures from Bayer AG and Blueprint Medicines; and travel grants from PharmaMar, Pfizer, Bayer AG, Novartis, and Lilly. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
García-Valverde, A., Rosell, J., Sayols, S. et al. E3 ubiquitin ligase Atrogin-1 mediates adaptive resistance to KIT-targeted inhibition in gastrointestinal stromal tumor. Oncogene 40, 6614–6626 (2021). https://doi.org/10.1038/s41388-021-02049-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-02049-0
This article is cited by
-
FBXW7 regulates the sensitivity of imatinib in gastrointestinal stromal tumors by targeting MCL1
Gastric Cancer (2024)
-
The microphthalmia-associated transcription factor is involved in gastrointestinal stromal tumor growth
Cancer Gene Therapy (2023)