Abstract
Study design
Feasibility and preliminary clinical efficacy analysis in a single-arm interventional study.
Objectives
We developed a brain–computer interface-triggered functional electrical stimulation therapy (BCI-FEST) system for clinical application and conducted an interventional study to (1) assess its feasibility and (2) understand its potential clinical efficacy for the rehabilitation of reaching and grasping in individuals with sub-acute spinal cord injury (SCI).
Setting
Spinal cord injury rehabilitation hospital—Toronto Rehabilitation Institute—Lyndhurst Centre.
Methods
Five participants with sub-acute SCI completed between 12 and 40 1-hour sessions using BCI-FEST, with up to 5 sessions a week. We assessed feasibility by measuring participants’ compliance with treatment, the occurrence of adverse events, BCI sensitivity, and BCI setup duration. Clinical efficacy was assessed using Functional Independence Measure (FIM) and Spinal Cord Independence Measure (SCIM), as primary outcomes. In addition, we used two upper-limb function tests as secondary outcomes.
Results
On average, participants completed 29.8 sessions with no adverse events. Only one of the 149 sessions was affected by technical challenges. The BCI sensitivity ranged between 69.5 and 80.2%, and the mean BCI setup duration was ~11 min. In the primary outcomes, three out of five participants showed changes greater than the minimal clinically important differences (MCIDs). Additionally, the mean change in secondary outcome measures met the threshold for detecting MCID as well; four out of five participants achieved MCID.
Conclusions
The new BCI-FEST intervention is safe, feasible, and promising for the rehabilitation of reaching and grasping after SCI.
Similar content being viewed by others
Introduction
Improving voluntary arm and hand motor function is a rehabilitation priority for individuals living with tetraplegia resulting from spinal cord injury (SCI) [1,2,3]. An important rehabilitation strategy for recovering voluntary upper extremity movement after SCI is functional electrical stimulation therapy (FEST), in which functional electrical stimulation (FES) is used to retrain motor functions, such as reaching and grasping [4,5,6,7,8]. FES employs controlled electrical pulses delivered over multiple channels to contract muscles in synergy and facilitate functional movements. Consequently, FES can help individuals with tetraplegia practice a range of purposeful movements (e.g., eating, drinking, writing), even in cases with severe impairment.
In conventional FEST, the therapist is responsible for activating the stimulation. However, the recent adoption of brain–computer interface (BCI) technology in the rehabilitation of voluntary movement has made it possible to activate the stimulation using brain signals. A BCI translates brain activity into a control signal and can provide real-time feedback on the status of motor-related activity during rehabilitation [9]. As such, a BCI enables direct communication between the patient and a device, in this case, a stimulator, without the need for external control. The first effort to combine BCI and FES for the restoration of upper extremity function in an individual with tetraplegia was conducted by Pfurtscheller et al. who presented a BCI-controlled FES system as a novel assistive device for grasping [10].
Since then, various researchers have attempted to refine the combination of BCI and FES technologies with a focus on its use as a therapeutic tool for rehabilitation after a stroke or SCI [11,12,13]. For example, Biasiucci et al. developed a 16 EEG-channel BCI system and coupled it with a single-channel FES system for facilitating hand opening [11]. Their study compared the effectiveness of BCI-FES to ‘sham-FES’ therapy for arm function recovery after stroke. Twenty-seven adults with chronic stroke received ten 1-hour sessions of BCI-FES (n = 14) or sham-FES (n = 13) therapy. The BCI-FES therapy included participants triggering the stimulation by attempting movements following a visual cue, while in ‘sham-FES’ therapy the stimulation was always delivered 3.5–5.5 s following the cue. The authors reported that BCI-FES elicited significant, clinically relevant, and lasting motor recovery in chronic stroke survivors more effectively than the sham FES.
A randomized pilot study by Osuagwu et al. compared the effects of a BCI-controlled FES and ‘passive FES’ on the neurological and functional outcomes in hospitalized patients with sub-acute SCI [12]. Passive FES in that study referred to stimulation activated automatically, following 10 s ON and 10 s OFF pattern. All 12 study participants received 20 1-hour treatment sessions. The BCI-FES group (n = 7) received active therapy, in which the BCI system, developed using 3 bipolar EEG channels (i.e., 6 electrodes), was used to trigger the stimulation following a successful detection of a movement attempt. In contrast, the FES group (n = 5) received passive FES therapy. Stimulation was intended to assist in hand opening and closing using a four-channel stimulator. The authors concluded that the effects of the BCI-FES group were greater than the FES group.
While these results are promising, the aforementioned systems used either BCI systems with multiple EEG channels or FES systems that facilitate only simple movements—both aspects can represent barriers that could limit the use of this technology in clinical practice. More specifically, the use of multiple EEG channels might take away valuable active treatment time allocated for a session. Hence, a small number of channels that can be set up quickly would be more desirable. Besides, FEST for upper extremity rehabilitation often incorporates practicing numerous complex movements requiring simultaneous multi-muscle stimulation, and hence an FES device supporting only a few hand movements might not be sufficient.
We developed a BCI system that uses a single EEG channel per hand (i.e., up to two channels for bimanual therapy), and designed it specifically to be integrated with FEST [14, 15]. The integration has resulted in a new rehabilitation strategy for upper extremity motor rehabilitation: BCI-controlled FEST (BCI-FEST). We conducted the present study to (1) investigate the feasibility of delivering BCI-FEST for the rehabilitation of reaching and grasping in adults with incomplete sub-acute tetraplegia resulting from cervical SCI, and (2) gain a preliminary understanding of the therapeutic effects of BCI-FEST in SCI.
Methods
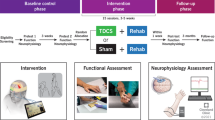
A single-arm interventional study is presented. All recruited participants were offered 40 sessions of BCI-FEST intervention to retrain the upper extremity function. Depending on the initial assessments and the participants’ goals, the intervention targeted either one or both upper extremities.
Participants
Adult individuals (i.e., 18 years of age or older), with traumatic incomplete SCI at the C4-C7 level, American Spinal Injury Association Impairment Scale (AIS) score of B–D, and less than 180 days post injury at the time of initial baseline assessment, were recruited for this study. All study participants provided written informed consent to join the study.
Materials
FES
We delivered FES using a four-channel programmable stimulator (Compex Motion, Switzerland) with self-adhesive transcutaneous electrodes [16]. The stimulator was programmed with multiple protocols to assist grasping, reaching, and reaching along with grasping movements. The stimulation consisted of bi-phasic asymmetrical pulses with a stimulation frequency of 40 Hz and a pulse width of 250 μs. The frequently stimulated muscles as well as examples of the functional movements which were practiced during therapy are presented in Table 1.
BCI
The BCI system used in this study is similar to the one previously reported by Marquez-Chin et al. [14]. The current BCI’s algorithm for detecting movement attempts is designed around event-related desynchronization (ERD), a decrease of power in a user-specific frequency band that is considered to be the result of decreased synchrony of the underlying neuronal population [17]. More importantly, ERD is present and can be detected during movement execution and attempts [18]. During a session, the BCI recorded EEG activity from a single channel at 200 Hz and processed it in real-time to detect decreases in power (i.e., reflecting movement attempts). EEG processing included band-pass filtering, squaring the signal to estimate the power, calculating the root mean square, and applying a moving average filter of 1 s in length (window size of 10 samples).
BCI calibration
In this study, we performed calibration for both hands since the rehabilitation in SCI targets both upper extremities. During the calibration session, the participants were comfortably seated in front of a monitor used for displaying graphical cues. They were asked to follow the cues: READY to prepare, GO to start attempting a hand movement, and STOP to stop moving and relax. This process was repeated at least 80 times with each hand.
During the calibration session, we recorded EEG signals from eight locations (F3, F4, Fz, C3, C1, C4, C2, and Cz of the 10–10 electrode placement system). After the recording, we segmented the EEG signals into 12-s long windows and aligned them to the GO experimental cue (8 s before the GO cue, and 4 s after). For each window, we generated spectrograms and averaged them to generate maps of estimated power changes across time and frequency (overlapping 2 Hz windows between 3 and 32 Hz with 1 Hz increments). Finally, we inspected the generated maps for each participant and selected electrode locations and frequency bands that exhibited a power decrease following the GO cue.
BCI-FEST integration
Two researchers delivered the BCI-FEST intervention: a licensed physical or occupational therapist and a BCI operator. At the beginning of each session, the therapist set up the FES system, while the BCI operator set up the BCI system. The FES system setup included identifying motor points and placing self-adhesive electrodes over the muscles needed to perform the selected movements. Once the electrodes were secured in place, the stimulation intensity was selected so that it would not be uncomfortable for the patient but still enough to produce a functional movement. Based on therapist’s expertise and practiced protocol in a given session, the setup time for the FEST system alone is commonly 5–10 min [6, 19].
The BCI setup included placing an electrode (or two electrodes if the session focused on bilateral upper extremities) over the position(s) determined during the BCI calibration and ensuring that their impedance value was below 10 kΩ. The time needed to set up the BCI system is discussed in more detail in the remainder of the article.
Intervention
During the session, the therapist had access to a foot pedal, which was used to set the BCI system in a “ready state” in which the brain activity could trigger the stimulation (see Fig. 1). Whenever the therapist asked the participant to attempt a movement, simultaneously he/she would press the foot pedal. The therapist could also use the foot pedal a second time to trigger the simulation if the BCI failed to detect the movement, enabling the continuation of therapy. The therapist was also responsible for guiding the arm and hand while the stimulation was active, ensuring the quality of the movement.
The EEG signals are recorded from a single channel—consisting of three electrodes—using an amplifier and a data acquisition card and processed using the laptop. When the therapist (left) asks the patient (right) to start attempting the movement, he/she activates a switch and enables the connection between the BCI and FES. If a decrease is detected in the BCI output (i.e., the participant’s processed EEG activity), the FES is triggered. Right: a flowchart with the sequence of events taking place during an FES protocol.
Participants received up to 40 1-hour BCI-FEST sessions with a maximum of five sessions delivered weekly. Additionally, all the participants received at least 3 hours of conventional occupational therapy (COT) per week during their stay in the hospital.
Historical comparison
In this feasibility report, we used a historical comparison to understand the potential efficacy of BCI-FEST on improving upper extremity motor function. The historical data were recorded as part of a randomized clinical trial conducted by Popovic et al. [6]. In that study, the effects of FEST were compared to the effects of COT for improving voluntary grasping in adults with sub-acute incomplete tetraplegia.
Outcome measures
Feasibility measures
To characterize the feasibility of the BCI-FEST intervention we recorded (i) the participants’ compliance with BCI-FEST treatment, (ii) the incidence of adverse events, (iii) BCI sensitivity, which was defined as the percentage of stimulation triggers achieved by the BCI, and (iv) setup duration, which was defined as the time needed for setting up the BCI system. The BCI sensitivity was calculated as the number of successful BCI triggers divided by the number of expected BCI triggers. The successful BCI trigger was defined as a BCI activation after the therapist had given the participant a cue to start attempting a movement. The BCI triggers were expected for every phase of the FES-assisted movements (e.g., opening a hand followed by closing it), except for the last phase, which turned off the stimulation. The therapists were instructed to manually activate the last trigger. This was done because, in the last phase of the movement, patients were asked to relax rather than attempt a movement.
Efficacy measures
We used the following clinical assessments to measure the change in the upper extremity function. Assessments were conducted at baseline, after 20 therapy sessions (midpoint), upon completion of 40 therapy sessions (discharge), and at 6 months after the baseline assessment (follow-up). Assessments at all timepoints were performed independently (using participants’ voluntary function) without the assistance of the FES.
Primary efficacy measures
The primary outcome measures were:
-
Functional independence measure (FIM)
-
Spinal cord independence measure (SCIM)
FIM and SCIM are self-reported assessments designed to capture the degree of disability and its effect on independent living [20,21,22]. Self-care components of FIM and SCIM were particularly important for this study, as most of the aspects being tested in these components require upper extremity function.
FIM total score ranges from 18 to 126 points, and higher scores indicate a greater level of independence. FIM self-care subscore ranges from 6 to 42 points.
Similarly, the SCIM total score ranges from 0 to 100 points, and higher scores indicate a greater level of independence. SCIM self-care subscore ranges from 0 to 20 points.
Secondary efficacy measures
We used the following assessments as secondary clinical measures:
-
3D Toronto Rehabilitation Institute—Hand Function Test (3D TRI-HFT)
-
Graded Redefined Assessment of Strength, Sensibility, and Prehension (GRASSP)
The 3D TRI-HFT is a 3D-printed clinical assessment tool to specifically measure the upper extremity gross motor function. The 3D-printed test has been recently validated in individuals with sub-acute SCI, whereas the original TRI-HFT was validated in SCI in 2012 by Kapadia et al. [23]. The test assesses the participant’s ability to manipulate ten 3D-printed everyday objects (e.g., mug, pencil, credit card). Each object is scored on a 0–7 points scale and higher scores indicate better performance.
The GRASSP is a quantitative clinical upper-limb impairment measure designed for use in SCI [24]. The five sections of GRASSP are: (i) strength (range: 0–50), (ii) sensibility-dorsal (range: 0–12), (iii) sensibility-palmar (range: 0–12), (iv) prehension-ability (range: 0–12), and (v) prehension-performance (range: 0–30). Each upper limb is scored independently, and higher scores indicate better function.
Results
Participants
We screened a total of 225 potential participants and identified 11 eligible individuals. Out of that group, five participants, with a mean (± standard deviation) age of 51.8 ± 17.5, were enrolled in the study. The average time since injury at enrollment was 71.4 days. Demographic information and the neurological descriptors of SCI at the time of the enrollment are provided in Table 2. The participants’ identifiers are not related to their names and/or initials.
Feasibility
Compliance with intervention
The participants completed 29.8 out of 40 therapy sessions on average, with a range of 12–40 sessions. More specifically, two out of five participants completed all 40 sessions. Two more participants completed close to 30 (29 and 28) sessions and one participant dropped out after 12 sessions (Table 3).
Adverse events and other issues
No adverse events were reported in 149 sessions across all participants. The BCI was successfully used to trigger the stimulation in 148 sessions, and in the single session for the single participant, we delivered conventional FEST, due to the technical problem with the hardware connection between BCI and FES systems.
BCI results
The electrode locations and frequency bands selected as BCI parameters are presented in Table 3. The BCI Sensitivity ranged between 69.5 and 80.2%. The average duration of the BCI setup was 11 min and 5 s across all participants (Table 3).
Efficacy
All five study participants completed the baseline assessments. Of the five participants, four completed the midpoint assessment (i.e., after 20 sessions), and one participant dropped out after completing 12 sessions, and hence for this participant midpoint assessment was done after 12 sessions. Three out of five participants completed the discharge assessment. None of the participants were available to come for the 6 months follow-up (this has been a common challenge for our team where out of 20 individuals on average we were able to get 5 participants for follow-up assessment [25]), so this measure has been omitted from the tables. The assessments were completed without the FES assistance, indicating that the observed changes, outlined below, persisted after the FES was no longer applied.
FIM
The mean change score on the FIM self-care sub-component from baseline to midpoint was 7.2 ± 6.67, and from baseline to discharge was 10.0 ± 8.16, for the three participants that completed the discharge assessment. More details on the individual participant self-care sub-scores on FIM and SCIM assessments are presented in Table 4. Table 4 also includes the means and standard deviations of the corresponding scores for the COT group from the historical data [6].
SCIM
The mean change score on the SCIM self-care sub-component from baseline to midpoint was 5.6 ± 5.38, and from baseline to discharge was 7.33 ± 5.73.
3D TRI-HFT
The 3D TRI-HFT was assessed separately for the left and right upper extremities. The mean change score on the Object Manipulation component for the left upper extremity from baseline to midpoint was 25.2 ± 20.41, and from baseline to discharge was 27.33 ± 23.11 for the three participants that completed the discharge assessment. For the right upper extremity, the mean change score on the Object Manipulation component from baseline to midpoint was 11.8 ± 13.73, and from baseline to discharge was 11.66 ± 4.78.
Details regarding the individual scores for all components of the 3D TRI-HFT are presented in Table 4, which also includes the means and standard deviations of the corresponding scores for the COT group from the historical data [6].
GRASSP
GRASSP assessment was also scored separately for each upper extremity. The Strength component scores increased across all five participants, with improvements ranging from 2 to 25 points. The individual participant GRASSP scores are presented in Table 5.
Discussion
In this study, we attempted to test the feasibility and preliminary efficacy of delivering BCI-FEST intervention for retraining upper extremity function in individuals with sub-acute incomplete SCI. We found that BCI-FEST intervention can be successfully carried out in individuals with SCI and that the setup required for carrying out the intervention required ~11 min, which is well within the time limits of setup for any conventional rehabilitation therapy. In terms of personnel requirements, two people were needed to carry out the session (including setup), which might exceed the available staff in many rehabilitation clinics. Whilst at the current stage, focused on feasibility testing, the BCI operator is required, future improvements to our system will aim to enable a trained clinician to deliver the BCI-FEST single-handedly.
EEG is used extensively for BCI development because it allows for noninvasive recording of electrical brain activity. However, in most BCI-FEST studies, the BCIs are designed to use multiple EEG channels, which prolongs the setup process and takes away time from therapy. Our single-channel design successfully resolved this issue, without compromising the sensitivity of the BCI in successfully triggering the stimulation. The average sensitivity of our BCI system was 74.52 ± 3.32% and exceeded the 70% threshold assumed to be a requirement for BCI applications to control feedback applications [26]. Another aspect in which our BCI-FEST system differs from most others is the complexity and variety of FES-assisted movements available for patients to practice during the intervention. To the best of our knowledge, this is the first BCI-FEST system that supports practicing multiple reaching and grasping movements during the rehabilitation of individuals with SCI.
With regards to participant compliance with treatment, we found that conducting 40 BCI therapy sessions at the rate of 3–5 sessions per week was challenging in the sub-acute inpatient SCI setting, due to two factors. First, we found that participants who were staying in the hospital had limited time outside their rehabilitation schedule to complete three to five sessions per week. Given that length of stay in the hospital where the study was conducted is on average 12–15 weeks for the targeted population and that it could take up to 2 weeks to screen and enroll the patients in the study, it was extremely challenging to complete a 12-week research program. Secondly, we found that participants who resided farther away from the hospital were more likely to discontinue on-site research participation due to transportation issues after their discharge.
Upon completion of therapy, three (OF, OG, and OI) out of five participants showed significant improvements in the self-reported FIM and SCIM scores. Importantly, the changes in FIM self-care sub-scores exceeded the minimal clinically important difference (MCID; MCID = 5.7 points) by 6.3, 4.3, and 14.3 points, for OF, OG, and OI, respectively [27]. Similarly, the changes in SCIM self-care sub-scores for the study participants exceeded the MCID value (2.8 points) by 6.2, 5.2, and 11.2 points for OF, OG, and OI, respectively [28].
The scores on the Object Manipulation component of the 3D TRI-HFT for both hands increased for all five participants, and in four of them, the changes were greater than 10% of the maximum score (i.e., 7 points), which has been used as a threshold for detecting MCID [27]. The same four participants experienced improvements on the Rectangular Blocks component of the 3D TRI-HFT, as well. A similar trend was also seen on the Strength component of the GRASSP assessment, where all participants showed improvement in both upper extremities, and for four of them, the changes were greater than the minimal detectable difference of 5 points [29].
In our comparison analysis with historical data, we focused on the COT group, due to the better match based on the level of injury with the participants from the present study. In this group, 11 out of 12 participants had an injury at the C4–C5 level, which was the same level as the participants in our study.
We found that, on average, our study participants showed higher scores or greater changes on all outcome measures compared to the COT group, indicating larger gains. This was particularly noticeable on the FIM and SCIM self-care sub-scores, as well as on the Object Manipulation score of the 3D TRI-HFT, shown in Fig. 2. Also shown in the same figure, the mean scores of FIM-SC, SCIM-SC, and 3D TRI-HFT Object Manipulation in the BCI-FEST group at midpoint were comparable to the mean scores in the historical COT group at discharge.
Top right: mean and standard deviation scores for the SCIM self-care component for the BCI-FEST and the historical COT groups; corresponding MCID is shown for scale. Bottom: mean scores and standard deviation for the Object Manipulation component for left upper extremity (L) and right upper extremity (R) of the 3D TRI-HFT for the BCI-FEST and the historical COT group; 10% of the maximal score is shown in the middle of the graph for scale.
Additionally, we compared the SCIM-SC scores of the BCI-FEST and COT groups against profiles of recovery (POR) for individuals with cervical SCI with AIS B and C classification from another study [30]. The profiles were created using SCIM data collected from eight centers in Ontario, Canada, without the description of the interventions. The data included five individuals with AIS B and 16 with AIS C, recorded at 4–6 weeks, 3 months, and 6 months post injury [30]. This comparison is summarized in Table 6.
In the present study, the assessment timepoints were determined based on the start of the intervention and, therefore, the time post injury at baseline, midpoint and discharge differed between participants. Because of that, we calculated the average time post injury across participants to quantify the descriptive timepoints.
The mean baseline SCIM-SC score for the BCI-FEST group was 1.8 at ~71 days post injury, which is two times lower than the mean SCIM-SC scores for the AIS B and C POR groups at ~30 days post injury. Moreover, the increase of 7.9 points in mean scores between baseline and discharge experienced by the BCI-FEST group after ~75 days is greater than the changes experienced in the AIS B and AIS C groups after ~150 days. Similar to the direct comparison against historical COT data, these results suggest a potentially increased efficacy (i.e., achieving a clinically important change in a shorter period) of BCI-FEST intervention during the sub-acute stage of rehabilitation.
We hypothesize that the efficacy of the BCI-triggered FEST stems from the massed repetition of practiced movements and three consecutively occurring events present in each practice iteration. The first event is the participant’s voluntary effort to trigger the stimulation by attempting a movement. The second event is the triggering of the stimulation based on brain activity. The third and final event is the participant receiving congruent somatosensory feedback in a form of an FES-assisted movement. This sequence of events is repeated more than 50 times in a single session, and several hundreds of times throughout the intervention.
Using EEG is not the only approach for allowing participants to voluntarily trigger stimulation during FEST for upper-limb rehabilitation. Using EMG, or hybrid EEG-EMG, approaches have been proposed as well [31,32,33]. However, the approaches using EMG signals assume that the participants can produce a reliable EMG response in their upper limb(s). This, unfortunately, is often not the case for individuals with more severe impairments. On the other hand, if EMG from a non-upper-limb muscle is used to trigger the stimulation, then the command and the feedback are misaligned. For example, if an eye blink is used to trigger the stimulation, then eye blink and hand movements are being coupled; two actions that are ordinarily not associated. In fact, EMG-triggered electrical stimulation did not improve arm function in more severely impaired individuals with chronic stroke [31]. Similarly, another study in the stroke population found no difference in the effects on upper-limb function between EMG-triggered and cyclic stimulation [32].
Limitations
The primary limitation of the present study is its small sample size (n = 5); hence, the study results should be interpreted with caution. The main focus of this report was feasibility testing, but since it is an interventional study, we recorded changes in function using clinical measures to get insight into the efficacy of BCI-FEST. In addition to using MCID values, we compared the current study results with historical data to better understand potential rehabilitation benefits. Nevertheless, larger studies are needed before stronger conclusions could be made with regards to the superiority of BCI-FEST over COT, or FEST.
The second limitation of this study is the absence of analysis on EMG interference, which has been documented to occur in EEG recordings [34]. During therapy, we did not record EMG making it impossible to precisely characterize the extent of interference of facial muscle activity on the recorded EEG signals during BCI-FEST. However, in each session, we took steps to minimize excessive shoulder, neck, and facial muscle activity in the periods preceding stimulation. The steps included: (i) therapist asking participants if they were ready before giving a cue to start attempting a movement, providing participants with an opportunity to get in a comfortable position; (ii) reminding participants to solely focus on attempting the movement following the therapist’s cue, until the stimulation is triggered. Moreover, we designed our BCI system to respond to a decrease in EEG power. Therefore, an EMG interference, which increases the signal power, could cause a missed BCI activation; an event that, if occurs, can be mitigated by the therapist’s manual switch.
Conclusion
In conclusion, we provided preliminary evidence of the feasibility and efficacy of BCI-FEST in individuals with sub-acute SCI. While it is feasible to carry out this therapy, we also found that the proposed intensity and duration of therapy might be a challenging factor at in-patient rehabilitation. All our study participants showed improvement post completion, however, larger studies with a control group designed within the study are warranted.
References
Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–83.
Simpson LA, Eng JJ, Hsieh JTC, Wolfe DL. the SCIRE research team. the health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma. 2012;29:1548–55.
Snoek GJ, IJzerman MJ, Hermens HJ, Maxwell D, Biering-Sorensen F. Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Spinal Cord. 2004;42:526–32.
Kapadia N, Zivanovic V, Popovic MR. Restoring voluntary grasping function in individuals with incomplete chronic spinal cord injury: pilot study. Top Spinal Cord Inj Rehabil. 2013;19:279–87.
Popovic MR, Thrasher TA, Adams ME, Takes V, Zivanovic V, Tonack MI. Functional electrical therapy: retraining grasping in spinal cord injury. Spinal Cord. 2006;44:143.
Popovic MR, Kapadia NM, Zivanovic V, Furlan JC, Craven CB, McGillivray C. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair. 2011;25:433–42.
Mangold S, Keller T, Curt A, Dietz V. Transcutaneous functional electrical stimulation for grasping in subjects with cervical spinal cord injury. Spinal Cord. 2005;43:1–13.
Rushton DN. Functional electrical stimulation and rehabilitation—an hypothesis. Med Eng Phys. 2003;25:75–8.
Daly JJ, Wolpaw JR. Brain–computer interfaces in neurological rehabilitation. Lancet Neurol. 2008;7:1032–43.
Pfurtscheller G, Müller GR, Pfurtscheller J, Gerner HJ, Rupp R. “Thought” - Control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci Lett. 2003;351:33–6.
Biasiucci A, Leeb R, Iturrate I, Perdikis S, Al-Khodairy A, Corbet T, et al. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat Commun. 2018;9:2421.
Osuagwu BCA, Wallace L, Fraser M, Vuckovic A. Rehabilitation of hand in subacute tetraplegic patients based on brain computer interface and functional electrical stimulation: a randomised pilot study. J Neural Eng. 2016;13:065002.
Tabernig CB, Lopez CA, Carrere LC, Spaich EG, Ballario CH. Neurorehabilitation therapy of patients with severe stroke based on functional electrical stimulation commanded by a brain computer interface. J Rehabil Assist Technol Eng. 2018;5:2055668318789280.
Marquez-Chin C, Marquis A, Popovic MR. EEG-triggered functional electrical stimulation therapy for restoring upper limb function in chronic stroke with severe hemiplegia. Case Rep. Neurol Med. 2016;2016:1–11.
Jovanovic LI, Kapadia N, Lo L, Zivanovic V, Popovic MR, Marquez-Chin C. Restoration of upper limb function after chronic severe hemiplegia: a case report on the feasibility of a brain-computer interface-triggered functional electrical stimulation therapy. Am J Phys Med Rehabil. 2020;99:e35–40.
Keller T, Popovic MR, Pappas IP, Müller P-Y. Transcutaneous functional electrical stimulator “Compex Motion.”. Artif Organs. 2002;26:219–23.
Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–57.
Lopez-Larraz E, Montesano L, Gil-Agudo A, Minguez J. Continuous decoding of movement intention of upper limb self-initiated analytic movements from pre-movement EEG correlates. J Neuroeng Rehabil. 2014;11:153.
Moineau B, Marquez-Chin C, Alizadeh-Meghrazi M, Popovic MR. Garments for functional electrical stimulation: design and proofs of concept. J Rehabil Assist Technol Eng. 2019;6. https://doi.org/10.1177/2055668319854340.
Oczkowski WJ, Barreca S. The functional independence measure: Its use to identify rehabilitation needs in stroke survivors. Arch Phys Med Rehabil. 1993;74:1291–4.
Catz A, Itzkovich M, Agranov E, Ring H, Tamir A. SCIM—spinal cord independence measure: a new disability scale for patients with spinal cord lesions. Spinal Cord. 1997;35:850.
Itzkovich M, Gelernter I, Biering-Sorensen F, Weeks C, Laramee MT, Craven BC, et al. The spinal cord independence measure (SCIM) version III: reliability and validity in a multi-center international study. Disabil Rehabil. 2007;29:1926–33.
Kapadia N, Zivanovic V, Verrier M, Popovic MR. Toronto rehabilitation institute–hand function test: assessment of gross motor function in individuals with spinal cord injury. Top Spinal Cord Inj Rehabil. 2012;18:167–86.
Kalsi-Ryan S, Curt A, Verrier MC, Fehlings MG. Development of the graded redefined assessment of strength, sensibility and prehension (GRASSP): reviewing measurement specific to the upper limb in tetraplegia. J Neurosurg Spine. 2012;17:65–76.
Kapadia NM, Zivanovic V, Furlan J, Craven BC, McGillivray C, Popovic MR. Functional electrical stimulation therapy for grasping in traumatic incomplete spinal cord injury: randomized control trial. Artif Organs. 2011;35:212–6.
Blankertz B, Losch F, Krauledat M, Dornhege G, Curio G, Müller K-R. The Berlin brain-computer interface: accurate performance from first-session in BCI-naive subjects. IEEE Trans Biomed Eng. 2008;55:2452–62.
Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil. 2008;89:1693–700.
Scivoletto G, Tamburella F, Laurenza L, Molinari M. The spinal cord independence measure: how much change is clinically significant for spinal cord injury subjects. Disabil Rehabil. 2013;35:1808–13.
Kalsi-Ryan S, Beaton D, Ahn H, Askes H, Drew B, Curt A, et al. Responsiveness, sensitivity, and minimally detectable difference of the graded and redefined assessment of strength, sensibility, and prehension, version 1.0. J Neurotrauma. 2015;33:307–14.
Kalsi-Ryan S, Beaton D, Curt A, Popovic MR, Verrier MC, Fehlings MG. Outcome of the upper limb in cervical spinal cord injury: Profiles of recovery and insights for clinical studies. J Spinal Cord Med. 2014;37:503–10.
von Lewinski F, Hofer S, Kaus J, Merboldt K-D, Rothkegel H, Schweizer R, et al. Efficacy of EMG-triggered electrical arm stimulation in chronic hemiparetic stroke patients. Restor Neurol Neurosci. 2009;27:189–97.
de Kroon JR, IJzerman MJ. Electrical stimulation of the upper extremity in stroke: cyclic versus EMG-triggered stimulation. Clin Rehabil. 2008;22:690–7.
Sarasola-Sanz A, Irastorza-Landa N, Lopez-Larraz E, Bibian C, Helmhold F, Broetz D, et al. A hybrid brain-machine interface based on EEG and EMG activity for the motor rehabilitation of stroke patients. IEEE Int Conf Rehabil Robot. 2017;2017:895–900.
Yilmaz G, Budan AS, Ungan P, Topkara B, Türker KS. Facial muscle activity contaminates EEG signal at rest: evidence from frontalis and temporalis motor units. J Neural Eng. 2019;16:066029
Acknowledgements
The authors would like to acknowledge the immense assistance of Alexandra Chen, Chandy Green, Cindy Gauthier, Debbie Hebert, Hikaru Yokoyama, Jaclyn Dawe, Parvin Eftekhar, and Sharmini Atputharaj in the conduct of this study.
Funding
This study was supported by a grant from the Ontario Neurotrauma Foundation (#2016-RHI-EEG-1020), in partnership with Praxis Spinal Cord Institute.
Author information
Authors and Affiliations
Contributions
LIJ contributed to analyzing and interpreting the BCI results, creating the figures and tables, and writing the report. NK, VZ, HJR, MA, CM, and SKR contributed to data analysis and interpretation and the writing of the report. MRP and CMC contributed to interpreting the results and provided feedback on the report.
Corresponding author
Ethics declarations
Conflict of interest
MRP is a co-founder and a director of MyndTec, a company that manufactures transcutaneous functional electrical stimulators, and SKR is CEO and founder of Neural Outcomes Consulting, a company that manufactures the GRASSP and TRI-HFT. None of the remaining authors have conflicts of interest.
Ethics statement
This study was approved by the University Health Network Research Ethics Board.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jovanovic, L.I., Kapadia, N., Zivanovic, V. et al. Brain–computer interface-triggered functional electrical stimulation therapy for rehabilitation of reaching and grasping after spinal cord injury: a feasibility study. Spinal Cord Ser Cases 7, 24 (2021). https://doi.org/10.1038/s41394-020-00380-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-020-00380-4
This article is cited by
-
Upper Limb Recovery in Cervical Spinal Cord Injury After a Brain-Computer Interface Controlled Functional Electrical Stimulation Intervention
Journal of Medical and Biological Engineering (2023)
-
Therapists’ perspectives on using brain-computer interface-triggered functional electrical stimulation therapy for individuals living with upper extremity paralysis: a qualitative case series study
Journal of NeuroEngineering and Rehabilitation (2022)
-
Soft robotics and functional electrical stimulation advances for restoring hand function in people with SCI: a narrative review, clinical guidelines and future directions
Journal of NeuroEngineering and Rehabilitation (2022)